

Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
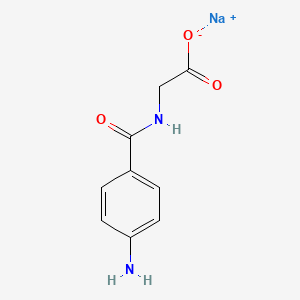

1. 4 Aminohippuric Acid
2. 4-aminohippuric Acid
3. Aminohippuric Acid
4. Nephrotest
5. P Aminohippurate
6. P Aminohippuric Acid
7. P-aminohippurate
8. P-aminohippuric Acid
9. Para Aminohippuric Acid
10. Para-aminohippurate, Sodium
11. Para-aminohippuric Acid
12. Sodium Para Aminohippurate
13. Sodium Para-aminohippurate
14. Sodium, Aminohippurate
1. 94-16-6
2. Sodium P-aminohippurate
3. P-aminohippurate Sodium
4. Sodium 4-aminohippurate
5. Monosodium P-aminohippurate
6. Aminohippurate (sodium)
7. Natrium 4-aminohippurat
8. Aminohippuric Acid Sodium Salt
9. P-aminohippuric Acid Sodium Salt
10. Sodium Para-aminohippurate
11. Aminohippurate
12. P-aminohippurate
13. N-(4-aminobenzoyl)glycine Monosodium Salt
14. Glycine, N-(4-aminobenzoyl)-, Monosodium Salt
15. Suo3kvs1o9
16. Aminohippurate Sodium [usp]
17. Sodium 4-amiropparaty Hyalrate
18. Chebi:31204
19. .rho.-aminohippuric Acid Sodium Salt
20. Sodium 4-aminohippurate Hydrate
21. Sodium;2-[(4-aminobenzoyl)amino]acetate
22. Aminohippurate Sodium (usp)
23. Ammohippurate Sodium
24. Sodium P-aminohippurate;p-aminohippuric Acid Sodium Salt
25. Amino-hippurate-sodium
26. Paraaminohippurate Injection
27. 4-aminohippursaeure, Natriumsalz
28. Einecs 202-309-8
29. Unii-suo3kvs1o9
30. Paraaminohippurate
31. Sodium Aminohippurate
32. Sodium 2-[(4-aminobenzoyl)amino]acetate
33. Paraaminohippurate (tn)
34. Hippuric Acid, P-amino-, Monosodium Salt
35. Aminohippurate [vandf]
36. 4-aminohippursaeure Natriumsalz
37. Schembl8964780
38. Sodium P-aminophippurate (jan)
39. Chembl1200365
40. 4-aminohippuric Acid Sodium Salt
41. Sodium (4-aminobenzamido)acetate
42. Dtxsid40916717
43. Sodium N-(4-aminobenzoyl)glycinate
44. Hy-a0080
45. Sodium 2-(4-aminobenzamido)acetate
46. Aminohippurate Sodium [vandf]
47. Sodium P-aminohippurate [jan]
48. Akos023093308
49. Akos024306942
50. Aminohippurate Sodium [who-dd]
51. Sodium [(4-aminobenzoyl)amino]acetate
52. Ccg-266692
53. Cs-4289
54. 4-aminohippuricacid,sodiumsaltmonohydrate
55. Aminohippurate Sodium [orange Book]
56. Aminohippurate Sodium [usp Impurity]
57. Sw219751-1
58. Para-aminohippurate Sodium [injection]
59. D01421
60. .rho.-aminohippuric Acid Sodium Salt [mi]
61. A844851
62. Q27114208
| Molecular Weight | 216.17 g/mol |
|---|---|
| Molecular Formula | C9H9N2NaO3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 216.05108644 g/mol |
| Monoisotopic Mass | 216.05108644 g/mol |
| Topological Polar Surface Area | 95.2 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 227 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Indicators and Reagents
Substances used for the detection, identification, analysis, etc. of chemical, biological, or pathologic processes or conditions. Indicators are substances that change in physical appearance, e.g., color, at or approaching the endpoint of a chemical titration, e.g., on the passage between acidity and alkalinity. Reagents are substances used for the detection or determination of another substance by chemical or microscopical means, especially analysis. Types of reagents are precipitants, solvents, oxidizers, reducers, fluxes, and colorimetric reagents. (From Grant and Hackh's Chemical Dictionary, 5th ed, p301, p499) (See all compounds classified as Indicators and Reagents.)
Market Place

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
