
Synopsis
Synopsis
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Afonilum
2. Aminodur
3. Aminophyllin
4. Aminophylline Df
5. Cardophyllin
6. Carine
7. Clonofilin
8. Corophyllin
9. Diaphyllin
10. Drafilyn
11. Duraphyllin
12. Ethylenediamine, Theophylline
13. Eufilina
14. Eufilina Venosa
15. Euphyllin
16. Euphyllin Retard
17. Euphylline
18. Godafilin
19. Mini-lix
20. Mundiphyllin
21. Mundiphyllin Retard
22. Novophyllin
23. Phyllocontin
24. Phyllotemp
25. Somophyllin
26. Tari-dog
27. Theophyllamin Jenapharm
28. Theophyllamine
29. Theophyllin Eda Ratiopharm
30. Theophyllin Eda-ratiopharm
31. Theophyllin Edaratiopharm
32. Theophylline Ethylenediamine
33. Truphylline
1. 317-34-0
2. Aminophyllin
3. Cardophyllin
4. Phyllocontin
5. Syntophyllin
6. Theophyllamine
7. Linampheta
8. Somophyllin
9. Truphylline
10. Theophylline Ethylenediamine
11. Aminodur
12. Aminocardol
13. Cardiofilina
14. Cardophylin
15. Diaphylline
16. Diuxanthine
17. Ethophylline
18. Eurphyllin
19. Genophyllin
20. Inophylline
21. Metaphyllin
22. Metaphylline
23. Methophylline
24. Neophyiline
25. Norofilina
26. Peterphyllin
27. Phylcardin
28. Phyllindon
29. Stenovasan
30. Theolamine
31. Theophyldine
32. Theophyllaminum
33. Thephyldine
34. Variaphylline
35. Vasofilina
36. Cardiomin
37. Cariomin
38. Diophllin
39. Euufillin
40. Grifomin
41. Lasodex
42. Lixaminol
43. Minaphil
44. Miofilin
45. Theodrox
46. Theolone
47. Carena
48. Aminophyllinum
49. Diaphilline
50. Diophyllin
51. Eufilina
52. Euphyllin
53. Euphylline
54. Euphyllinum
55. Euufilin
56. Theomin
57. Theophyllaminium
58. Dobo
59. Rectalad Aminophylline
60. Theophyline Ethylenediamine
61. Aminodur Dura-tabs
62. Theophyllin Aethylendiamin
63. Ammophyllin
64. Tefamin
65. Aminofilina
66. Aminofillina
67. Somophyllin O
68. Aminophylline Ethylenediamine
69. Rectalad-aminophylline
70. Theophyllin Ethylenediamine
71. Dura-tab S.m. Aminophylline
72. Etilen-xantisan Tabl.
73. By 108
74. Th/100
75. 1h-purine-2,6-dione, 3,7-dihydro-1,3-dimethyl-, Compd. With 1,2-ethanediamine (2:1)
76. Theophylline Compound With Ethylenediamine
77. Theophylline, Compd With Ethylenediamine (2:1)
78. Ethylenediamine, Compd. With Theophylline (1:2)
79. Theophylline-ethylenediamine Anhydrous
80. Theophylline Compound With Ethylenediamine (2:1)
81. Aminophylline (truphylline)
82. Theophylline-ethylenediamine
83. 27y3kjk423
84. Nsc-7919
85. Theophylline, Compd. With Ethylenediamine (2:1)
86. Aminophylline Anhydrous
87. Aminophylline Dye Free
88. 1,3-dimethyl-3,7-dihydro-1h-purine-2,6-dione Compound With Ethane-1,2-diamine (2:1)
89. Eufilina [polish]
90. Aminofillina [dcit]
91. Aminofilina [spanish]
92. Somopphyllin
93. Dura-tab Sm Aminophylline
94. 1,3-dimethyl-3,7-dihydro-1h-purine-2,6-dione Hemiethane-1,2-diamine Salt
95. Aminofilina [inn-spanish]
96. Aminophyllinum [inn-latin]
97. Pulmophyllin (new)
98. Mudrane Gg Tablets
99. Pulmophylline (new)
100. Hsdb 221
101. Theophyllinum Et Ethylendiaminum
102. Mudrane Gg-2 Tablets
103. Theophyllin Aethylendiamin [german]
104. Nsc 7919
105. Einecs 206-264-5
106. Unii-27y3kjk423
107. Etilen-xantisan
108. Aminophylline In Sodium Chloride 0.45%
109. Prestwick_93
110. Somophyllin (tn)
111. Aminophylline [usp:inn:ban:jan]
112. Mfcd00013221
113. 1,3-dimethyl-7h-purine-2,6-dione; Ethane-1,2-diamine
114. Ammonium Carbonate Nf
115. Aminophylline, Anhydrous
116. Aminophylline In Sodium Chloride 0.45% In Plastic Container
117. Lopac-a-1755
118. Aminophylline (usp/inn)
119. Aminophylline [mi]
120. Aminophylline [inn]
121. Schembl5037
122. 1h-purine-2,6-dione, 3,7-dihydro-1,3-dimethyl-, Compd With 1,2-ethanediamine (2:1)
123. 3,7-dihydro-1,3-dimethyl-1h-purine-2,6-dione Compd. With 1,2-ethanediamine (2:1)
124. Aminophylline [hsdb]
125. Aminophylline [inci]
126. Aminophylline [vandf]
127. Aminophylline [mart.]
128. Aminophylline Anhydrous Powder
129. Aminophylline [who-dd]
130. Aminophylline [who-ip]
131. Chebi:2659
132. Aminophylline, >=98%, Powder
133. Chembl1370561
134. Dtxsid40883359
135. Theophylline Ethylenediamine (tn)
136. Hms1570d21
137. Hms2097d21
138. Hms3260c09
139. Hms3655a19
140. Hms3714d21
141. Hms3884g06
142. Aminophylline [orange Book]
143. Bcp23387
144. Hy-b0140
145. Tox21_500014
146. Aminophylline [usp Monograph]
147. S1673
148. Aminophyllinum [who-ip Latin]
149. Akos015951253
150. Akos015960458
151. Theophylline And Ethylenediamine
152. Bcp9000293
153. Ccg-220820
154. Ccg-221318
155. Cs-1936
156. Db01223
157. Ks-5340
158. Lp00014
159. Aminophylline(phyllocontin, Truphylline)
160. Ncgc00016113-01
161. Ncgc00016113-02
162. Ncgc00016113-03
163. Ncgc00093539-01
164. Ncgc00260699-01
165. 95646-60-9
166. Ac-11138
167. Ba166016
168. Cas-317-34-0
169. Bcp0726000247
170. A2805
171. Eu-0100014
172. Ft-0622295
173. Sw199286-2
174. A 1755
175. D00227
176. I10006
177. A875823
178. Q471763
179. Sr-01000872628
180. Theophylline-ethylenediamine [ep Monograph]
181. Sr-01000872628-1
182. W-106881
183. Theophylline-ethylenediamine Anhydrous [ep Impurity]
184. 1,3-dimethyl-3,7-dihydro-1h-purine-2,6-dionehemiethane-1,2-diaminesalt
185. 1,2-ethanediamine, Compd. With 3,7-dihydro-1,3-dimethyl-1h-purine-2,6-dione (1:2)
186. 1,3-dimethyl-1h-purine-2,6(3h,7h)-dione Compound With Ethane-1,2-diamine (2:1)
187. 1,3-dimethyl-3,7-dihydro-1h-purine-2,6-dione - Ethane-1,2-diamine (2:1)
188. 1,3-dimethyl-3,9-dihydro-1h-purine-2,6-dione Compound With Ethane-1,2-diamine (2:1)
189. 1h-purine-2,6-dione, 3,9-dihydro-1,3-dimethyl-, Compd. With 1,2-ethanediamine (2:1)
190. 3,7-dihydro-1,3-dimethyl-1h-purine-2,6-dione, Compd With 1,2-ethanediamine (2:1)
191. 1h-purine-2,6-dione, 3,7-dihydro-1,3-dimethyl-, Compd. With 1,2-ethanediamine (2:1), Mixt. With 3,7-dihydro-1,3-dimethyl-1h-purine-2,6-dione
| Molecular Weight | 420.43 g/mol |
|---|---|
| Molecular Formula | C16H24N10O4 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 1 |
| Exact Mass | 420.19819929 g/mol |
| Monoisotopic Mass | 420.19819929 g/mol |
| Topological Polar Surface Area | 191 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 273 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 2 | |
|---|---|
| Drug Name | Aminophylline |
| PubMed Health | Aminophylline |
| Drug Classes | Bronchodilator, Theophylline |
| Drug Label | Aminophylline is a 2:1 complex of theophylline and ethylenediamine. Theophylline is structurally classified as a methyxanthine. Aminophylline occurs as a white or slightly yellowish granule or powder, with a slight ammoniacal odor. Aminophylline has... |
| Active Ingredient | Aminophylline |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 25mg/ml |
| Market Status | Prescription |
| Company | Hospira; Luitpold |
| 2 of 2 | |
|---|---|
| Drug Name | Aminophylline |
| PubMed Health | Aminophylline |
| Drug Classes | Bronchodilator, Theophylline |
| Drug Label | Aminophylline is a 2:1 complex of theophylline and ethylenediamine. Theophylline is structurally classified as a methyxanthine. Aminophylline occurs as a white or slightly yellowish granule or powder, with a slight ammoniacal odor. Aminophylline has... |
| Active Ingredient | Aminophylline |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 25mg/ml |
| Market Status | Prescription |
| Company | Hospira; Luitpold |
Bronchodilator Agents; Cardiotonic Agents; Phosphodiesterase Inhibitors; Purinergic P1 Receptor Antagonists
National Library of Medicine's Medical Subject Headings. Aminophylline. Online file (MeSH, 2016). Available from, as of June 24, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Aminophylline is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of July 6, 2016: https://clinicaltrials.gov/search/intervention=Aminophylline
IV theophylline (often as aminophylline) has been used to relieve the periodic apnea and increase arterial blood pH in patients with Cheyne-Stokes respiration. /NOT included in US product labeling/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3602
MEDICATION (VET): Aminophylline is indicated for control of reversible airway constriction, to prevent bronchoconstriction, and as an adjunct with other respiratory disease treatment. The uses are are similar to the indications for theophylline because it is a salt form of theophylline. It is used for inflammatory airway disease in cats (feline asthma), dogs, and horses. In dogs, the uses include collapsing trachea, bronchitis, and other airway disease. It has not been effective for respiratory diseases in cattle.
Papich, M.G. Saunders Handbook of Veterinary Drugs Small and Large Animal. 3rd ed. St. Louis, MO: Elsevier Saunders, 2011, p. 28
For more Therapeutic Uses (Complete) data for AMINOPHYLLINE (10 total), please visit the HSDB record page.
Fatalities in adults have generally occurred during or following IV administration of large doses of aminophylline in patients with renal, hepatic, or cardiovascular complications. In other patients, the rapidity of the injection, rather than the dose used, appears to be the more important factor precipitating acute hypotension, seizures, coma, cardiac standstill, ventricular fibrillation, and death. IV aminophylline or theophylline should therefore be given slowly. In children, fatalities usually are a result of overdosage and marked sensitivity to the CNS stimulation of theophylline.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3606
When administered rectally as suppositories (dosage form no longer commercially available in the US), theophyllines have caused rectal irritation and inflammation. /Theophyllines/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3605
Rapid IV injection of aminophylline may produce dizziness, faintness, lightheadedness, palpitation, syncope, precordial pain, flushing, profound bradycardia, ventricular premature complexes (VPCs, PVCs), severe hypotension, or cardiac arrest. IM injection of aminophylline produces intense local pain and sloughing of tissue ... .
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3605
Theophyllines may also produce transiently increased urinary frequency, dehydration, twitching of fingers and hands, tachypnea, and elevated serum AST (SGOT) concentrations. Hypersensitivity reactions characterized by urticaria, generalized pruritus, and angioedema have been reported with aminophylline administration. A contact-type dermatitis, caused by hypersensitivity to the ethylenediamine component of aminophylline, has also been reported. Bone marrow suppression, leukopenia, thrombocytopenia, and hemorrhagic diathesis have also been reported, but their association with theophylline therapy is questionable. Other adverse effects of theophyllines include albuminuria, increased urinary excretion of renal tubular cells and erythrocytes, hyperglycemia, and syndrome of inappropriate secretion of antidiuretic hormone (SIADH). /Theophyllines/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3605
For more Drug Warnings (Complete) data for AMINOPHYLLINE (22 total), please visit the HSDB record page.
For the treatment of bronchospasm due to asthma, emphysema and chronic bronchitis.
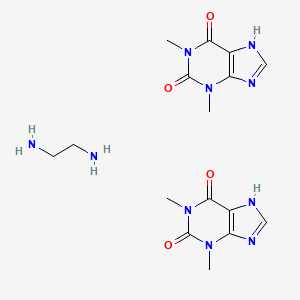
Aminophylline is the ethylenediamine salt of theophylline. Theophylline stimulates the CNS, skeletal muscles, and cardiac muscle. It relaxes certain smooth muscles in the bronchi, produces diuresis, and causes an increase in gastric secretion.
Cardiotonic Agents
Agents that have a strengthening effect on the heart or that can increase cardiac output. They may be CARDIAC GLYCOSIDES; SYMPATHOMIMETICS; or other drugs. They are used after MYOCARDIAL INFARCT; CARDIAC SURGICAL PROCEDURES; in SHOCK; or in congestive heart failure (HEART FAILURE). (See all compounds classified as Cardiotonic Agents.)
Bronchodilator Agents
Agents that cause an increase in the expansion of a bronchus or bronchial tubes. (See all compounds classified as Bronchodilator Agents.)
Phosphodiesterase Inhibitors
Compounds which inhibit or antagonize the biosynthesis or actions of phosphodiesterases. (See all compounds classified as Phosphodiesterase Inhibitors.)
Purinergic P1 Receptor Antagonists
Compounds that bind to and block the stimulation of PURINERGIC P1 RECEPTORS. (See all compounds classified as Purinergic P1 Receptor Antagonists.)
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03D - Other systemic drugs for obstructive airway diseases
R03DA - Xanthines
R03DA05 - Aminophylline
Volume of Distribution
0.3 to 0.7 L/kg
Clearance
0.29 mL/kg/min [postnatal age 3-15 days]
0.64 mL/kg/min [postnatal age 25-57 days]
1.7 mL/kg/min [1-4 years]
1.6 mL/kg/min [4-12 years]
0.9 mL/kg/min [13-15 years]
1.4 mL/kg/min [16-17 years]
0.65 mL/kg/min [Adults (16-60 years), non-smoking asthmatics]
0.41 mL/kg/min [Elderly (>60 years). liver, and renal function]
0.33 mL/kg/min [Acute pulmonary edema]
0.54 mL/kg/min [COPD->60 years, stable non-smoker >1 year]
0.48 mL/kg/min [COPD with cor pulmonale]
1.25 mL/kg/min [Cystic fibrosis (14-28 years)]
0.31 mL/kg/min [Liver disease -cholestasis]
0.35 mL/kg/min [cirrhosis]
0.65 mL/kg/min [acute hepatitis]
0.47 mL/kg/min [Sepsis with multi-organ failure]
0.38 mL/kg/min [hypothyroid]
0.8 mL/kg/min [hyperthyroid]
IV theophylline produces the highest and most rapid serum theophylline concentration. Following a single IV dose of theophylline (as aminophylline) of about 5 mg/kg over 30 minutes to healthy adults, mean peak serum theophylline concentrations of about 10 ug/mL are reached.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3607
In neonates, approximately 50% of the theophylline dose is excreted unchanged in the urine. Beyond the first three months of life, approximately 10% of the theophylline dose is excreted unchanged in the urine. /Theophylline/
NIH; DailyMed. Current Medication Information for Aminophylline (Aminophylline Dihydrate) Tablet (Updated: March 2012). Available from, as of July 22, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4bc5d8ca-58de-4683-a27a-3e216b20e82b
When administered IM, theophylline is usually absorbed slowly and incompletely. Rectal suppositories (no longer commercially available in the US) are slowly and erratically absorbed, regardless of whether the suppository base is hydrophilic or lipophilic. /Theophylline/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3607
Dissolution appears to be the rate-limiting step in the absorption of oral theophylline. Under the acidic conditions of the stomach, the theophylline salts and compounds release free theophylline. ... Microcrystalline dosage forms and oral solutions of theophyllines are absorbed more rapidly, but not to a greater extent, than are uncoated tablets. Although the rate of absorption is slower, extended-release preparations (capsules and tablets) of theophylline are generally absorbed to the same extent as uncoated tablets; however, the actual rate of absorption of extended-release preparations may differ. Extended-release preparations of theophyllines have been formulated to release the drug at various rates suitable for dosing every 8-12, 12, or 24 hours; however, the actual dosing frequency for a given patient depends on their individual pharmacokinetic parameters. Since the rate and extent of absorption may differ between various extended-release preparations and sometimes between different dosage sizes of the same preparation, patients should generally be stabilized on a given preparation; substitution of one extended-release preparation for another should generally only be made when the preparations have been shown to be equivalent and/or the patient is evaluated pharmacokinetically during the transition period. Absorption of theophyllines may also be delayed, but is generally not reduced, by the presence of food in the GI tract; however, the effect of food on the absorption of extended-release preparations appears to be variable, and the manufacturer's recommendations for administration of specific preparations should be followed. /Theophyllines/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3607
For more Absorption, Distribution and Excretion (Complete) data for AMINOPHYLLINE (12 total), please visit the HSDB record page.
Both the N-demethylation and hydroxylation pathways of theophylline biotransformation are capacity-limited. Due to the wide intersubject variability of the rate of theophylline metabolism, non-linearity of elimination may begin in some patients at serum theophylline concentrations <10 mcg/mL. Since this non-linearity results in more than proportional changes in serum theophylline concentrations with changes in dose, it is advisable to make increases or decreases in dose in small increments in order to achieve desired changes in serum theophylline concentrations. Accurate prediction of dose-dependency of theophylline metabolism in patients a priori is not possible, but patients with very high initial clearance rates (i.e., low steady-state serum theophylline concentrations at above average doses) have the greatest likelihood of experiencing large changes in serum theophylline concentration in response to dosage changes. /Theophylline/
NIH; DailyMed. Current Medication Information for Aminophylline (Aminophylline Dihydrate) Tablet (Updated: March 2012). Available from, as of July 22, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4bc5d8ca-58de-4683-a27a-3e216b20e82b
Caffeine and 3-methylxanthine are the only theophylline metabolites with pharmacologic activity. 3-methylxanthine has approximately one tenth the pharmacologic activity of theophylline and serum concentrations in adults with normal renal function are <1 ug/mL. In patients with end-stage renal disease, 3-methylxanthine may accumulate to concentrations that approximate the unmetabolized theophylline concentration. Caffeine concentrations are usually undetectable in adults regardless of renal function. In neonates, caffeine may accumulate to concentrations that approximate the unmetabolized theophylline concentration and thus, exert a pharmacologic effect. /Theophylline/
NIH; DailyMed. Current Medication Information for Aminophylline (Aminophylline Dihydrate) Tablet (Updated: March 2012). Available from, as of July 22, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4bc5d8ca-58de-4683-a27a-3e216b20e82b
Theophylline is metabolized by the liver to 1,3-dimethyluric acid, 1-methyluric acid, and 3-methylxanthine. ... Individuals metabolize theophylline at different rates; however, individual metabolism of the drug is generally reproducible. Theophylline and its metabolites are excreted mainly by the kidneys. Renal clearance of the drug, however, contributes only 8-12% of the overall plasma clearance of theophylline. Small amounts of theophylline are excreted in feces unchanged. /Theophylline/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3607
Theophylline is metabolized via the microsomal cytochrome p450 system, primarily by the isozyme CYP1A2. The major pathway is demethylation to 3-methylxanthine in addition to being demethylated or oxidized to other metabolites. Less than 10% of theophylline is excreted in the urine unchanged. /Theophylline/
Goldfrank, L.R., Goldfrank's Toxicologic Emergencies 9th Ed. 2011., McGraw-Hill, New York, N.Y., p. 954
Following oral dosing, theophylline does not undergo any measurable first-pass elimination. In adults and children beyond one year of age, approximately 90% of the dose is metabolized in the liver. Biotransformation takes place through demethylation to 1-methylxanthine and 3-methylxanthine and hydroxylation to 1,3-dimethyluric acid. 1-methylxanthine is further hydroxylated, by xanthine oxidase, to 1-methyluric acid. About 6% of a theophylline dose is N-methylated to caffeine. Theophylline demethylation to 3-methylxanthine is catalyzed by cytochrome P-450 1A2, while cytochromes P-450 2E1 and P-450 3A3 catalyze the hydroxylation to 1,3-dimethyluric acid. Demethylation to 1-methylxanthine appears to be catalyzed either by cytochrome P-450 1A2 or a closely related cytochrome. In neonates, the N-demethylation pathway is absent while the function of the hydroxylation pathway is markedly deficient. The activity of these pathways slowly increases to maximal levels by one year of age. /Theophylline/
NIH; DailyMed. Current Medication Information for Aminophylline (Aminophylline Dihydrate) Tablet (Updated: March 2012). Available from, as of July 22, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4bc5d8ca-58de-4683-a27a-3e216b20e82b
7-9 hours
Theophylline clearance rates and half-life values were measured in 15 infants aged three to 23 months, after infusion of aminophylline by the intravenous route for at least 24 hours. ... The mean half-life was 4.4 +/- 2.2 hours. There was a tenfold variability in half-life, suggesting that individualization of theophylline dose is especially important in infants if undertreatment and toxicity are to be avoided.
PMID:711929 Simons FE, Simons KJ; J Clin Pharmacol 18 (10): 472-6 (1978)
Aminophylline is the ethylenediamine salt of theophylline. After ingestion, theophylline is released from aminophylline, and theophylline relaxes the smooth muscle of the bronchial airways and pulmonary blood vessels and reduces airway responsiveness to histamine, methacholine, adenosine, and allergen. Theophylline competitively inhibits type III and type IV phosphodiesterase (PDE), the enzyme responsible for breaking down cyclic AMP in smooth muscle cells, possibly resulting in bronchodilation. Theophylline also binds to the adenosine A2B receptor and blocks adenosine mediated bronchoconstriction. In inflammatory states, theophylline activates histone deacetylase to prevent transcription of inflammatory genes that require the acetylation of histones for transcription to begin.
Theophylline and aminophylline have been widely used as inhibitors of phosphodiesterase when examining the role of cAMP in regulating cell function. In reality, however, these phosphodiesterase inhibitors may have additional sites of action that could complicate the interpretation of the results. These additional sites of action could include antagonism of inhibitory adenosine autoreceptors and release of intracellular calcium. The purpose of the present study was to determine which of the above three is the primary mechanism by which theophylline and aminophylline affect transmitter release at the mammalian neuromuscular junction. Quantal release measurements were made using intracellular recording techniques. A variety of drugs were used to elucidate this pathway. Isoproterenol, an adenylate cyclase activator, was first used to establish the effect of enhanced levels of cAMP. Theophylline application on its own or in the presence of a drug combination that blocked the adenosine receptor and phosphodiesterase pathways caused significant release depression, opposite to what is expected if it was functioning to enhance cAMP levels. However, when applied in the presence of a drug combination that blocked the adenosine receptor, phosphodiesterase and intracellular ryanodine calcium pathways, theophylline was unable to depress release. Therefore, it was concluded that the major mechanism of action of theophylline is depression of transmitter release by causing the release of intracellular calcium. Aminophylline application alone resulted in a significant enhancement of release. However, when coupled with an adenosine receptor blocker, the ability of aminophylline to enhance transmitter release was blocked, suggesting that its dominant mechanism of action is adenosine receptor inhibition. Taken together, these results indicate that the use of theophylline and aminophylline is inappropriate when examining the role of cAMP at the mammalian neuromuscular junction.
PMID:16700879 Nickels TJ et al; Clin Exp Pharmacol Physiol 33 (5-6): 465-70 (2006)
Theophylline has two distinct actions in the airways of patients with reversible obstruction; smooth muscle relaxation (i.e., bronchodilation) and suppression of the response of the airways to stimuli (i.e., non-bronchodilator prophylactic effects). While the mechanisms of action of theophylline are not known with certainty, studies in animals suggest that bronchodilatation is mediated by the inhibition of two isozymes of phosphodiesterase (PDE III and, to a lesser extent, PDE IV) while non-bronchodilator prophylactic actions are probably mediated through one or more different molecular mechanisms, that do not involve inhibition of PDE III or antagonism of adenosine receptors. Some of the adverse effects associated with theophylline appear to be mediated by inhibition of PDE III (e.g., hypotension, tachycardia, headache, and emesis) and adenosine receptor antagonism (e.g., alterations in cerebral blood flow). Theophylline increases the force of contraction of diaphragmatic muscles. This action appears to be due to enhancement of calcium uptake through an adenosine-mediated channel. /Theophylline/
NIH; DailyMed. Current Medication Information for Aminophylline (Aminophylline Dihydrate) Tablet (Updated: March 2012). Available from, as of July 22, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4bc5d8ca-58de-4683-a27a-3e216b20e82b

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
45
PharmaCompass offers a list of Aminophylline API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Aminophylline manufacturer or Aminophylline supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Aminophylline manufacturer or Aminophylline supplier.
PharmaCompass also assists you with knowing the Aminophylline API Price utilized in the formulation of products. Aminophylline API Price is not always fixed or binding as the Aminophylline Price is obtained through a variety of data sources. The Aminophylline Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Aminophylline manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Aminophylline, including repackagers and relabelers. The FDA regulates Aminophylline manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Aminophylline API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Aminophylline manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Aminophylline supplier is an individual or a company that provides Aminophylline active pharmaceutical ingredient (API) or Aminophylline finished formulations upon request. The Aminophylline suppliers may include Aminophylline API manufacturers, exporters, distributors and traders.
click here to find a list of Aminophylline suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Aminophylline DMF (Drug Master File) is a document detailing the whole manufacturing process of Aminophylline active pharmaceutical ingredient (API) in detail. Different forms of Aminophylline DMFs exist exist since differing nations have different regulations, such as Aminophylline USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Aminophylline DMF submitted to regulatory agencies in the US is known as a USDMF. Aminophylline USDMF includes data on Aminophylline's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Aminophylline USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Aminophylline suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Aminophylline Drug Master File in Japan (Aminophylline JDMF) empowers Aminophylline API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Aminophylline JDMF during the approval evaluation for pharmaceutical products. At the time of Aminophylline JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Aminophylline suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Aminophylline Drug Master File in Korea (Aminophylline KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Aminophylline. The MFDS reviews the Aminophylline KDMF as part of the drug registration process and uses the information provided in the Aminophylline KDMF to evaluate the safety and efficacy of the drug.
After submitting a Aminophylline KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Aminophylline API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Aminophylline suppliers with KDMF on PharmaCompass.
A Aminophylline CEP of the European Pharmacopoeia monograph is often referred to as a Aminophylline Certificate of Suitability (COS). The purpose of a Aminophylline CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Aminophylline EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Aminophylline to their clients by showing that a Aminophylline CEP has been issued for it. The manufacturer submits a Aminophylline CEP (COS) as part of the market authorization procedure, and it takes on the role of a Aminophylline CEP holder for the record. Additionally, the data presented in the Aminophylline CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Aminophylline DMF.
A Aminophylline CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Aminophylline CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Aminophylline suppliers with CEP (COS) on PharmaCompass.
A Aminophylline written confirmation (Aminophylline WC) is an official document issued by a regulatory agency to a Aminophylline manufacturer, verifying that the manufacturing facility of a Aminophylline active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Aminophylline APIs or Aminophylline finished pharmaceutical products to another nation, regulatory agencies frequently require a Aminophylline WC (written confirmation) as part of the regulatory process.
click here to find a list of Aminophylline suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Aminophylline as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Aminophylline API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Aminophylline as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Aminophylline and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Aminophylline NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Aminophylline suppliers with NDC on PharmaCompass.
Aminophylline Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Aminophylline GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Aminophylline GMP manufacturer or Aminophylline GMP API supplier for your needs.
A Aminophylline CoA (Certificate of Analysis) is a formal document that attests to Aminophylline's compliance with Aminophylline specifications and serves as a tool for batch-level quality control.
Aminophylline CoA mostly includes findings from lab analyses of a specific batch. For each Aminophylline CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Aminophylline may be tested according to a variety of international standards, such as European Pharmacopoeia (Aminophylline EP), Aminophylline JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Aminophylline USP).
