
Synopsis
Synopsis
0
VMF
DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Amiobeta
2. Amiodarex
3. Amiodarona
4. Amiodarone
5. Amiohexal
6. Aratac
7. Braxan
8. Corbionax
9. Cordarex
10. Cordarone
11. Hydrochloride, Amiodarone
12. Kordaron
13. L 3428
14. L-3428
15. L3428
16. Ortacrone
17. Rytmarone
18. Skf 33134 A
19. Skf 33134-a
20. Skf 33134a
21. Tachydaron
22. Trangorex
1. 19774-82-4
2. Amiodarone Hcl
3. Nexterone
4. Amiodar
5. Pacerone
6. Ritmocardyl
7. Trangorex
8. Amiodarone (hydrochloride)
9. Uro-septra
10. Miodaron
11. Ortacrone
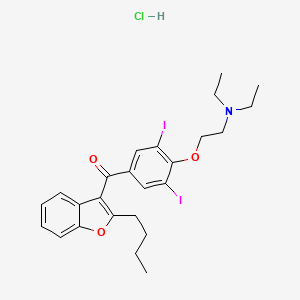
12. (2-butylbenzofuran-3-yl)(4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl)methanone Hydrochloride
13. Skf 33134-a
14. 51087 N Hcl
15. Nsc-85442
16. Mls000028520
17. Amiodarone Hydrochloride [jan]
18. Skf-33134-a
19. Methanone,(2-butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]-,hydrochloride
20. 976728sy6z
21. Smr000058296
22. L-3428
23. 2-butyl-3-benzofuranyl 4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl Ketone Hydrochloride
24. 2-butyl-3-benzofuryl 4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl Ketone Hydrochloride
25. Dsstox_cid_17185
26. Dsstox_rid_79304
27. Dsstox_gsid_37185
28. (2-butyl-1-benzofuran-3-yl)-[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone;hydrochloride
29. Rythmarone
30. Angoron
31. Atlansil
32. Miodrone
33. Renodoron
34. Amiodaronum Hydrochloride
35. L 3428 Labaz
36. Sr-01000003087
37. Ncgc00015096-12
38. Cas-19774-82-4
39. Ancaron
40. Amio-aqueous
41. Unii-976728sy6z
42. Hsdb 6525
43. Cordarone (tn)
44. Nexterone (tn)
45. Prestwick_707
46. Amiodarone Hydrochloride [usp:jan]
47. Ancaron (tn)
48. Einecs 243-293-2
49. Skf 33134 A
50. Mfcd00069204
51. Nsc 85442
52. Amiodaronehydrochloride
53. Cpd000058296
54. Opera_id_568
55. Schembl41348
56. Mls001076313
57. Mls001424272
58. Mls002222247
59. Spectrum2300165
60. Chebi:2664
61. Amiodarone Hydrochloride Solution
62. Chembl1083993
63. Dtxsid7037185
64. Amiodarone Hydrochloride - Bio-x
65. Calcium Channel (l Type) Blocker
66. Hms1569a20
67. Pharmakon1600-02300165
68. Act02681
69. Amiodarone Hydrochloride, >/=98%
70. Bcp13645
71. Nsc85442
72. Ketone,5-diiodophenyl, Hydrochloride
73. Tox21_113478
74. Tox21_300395
75. Tox21_500122
76. Ac-078
77. Amiodarone Hydrochloride [mi]
78. Ccg-39607
79. Nsc759560
80. Pm-101
81. S1979
82. Sk&f-33134-a
83. Amiodarone Hydrochloride (jp17/usp)
84. Akos015844018
85. Amiodarone Hydrochloride [hsdb]
86. Cs-1371
87. Ks-5249
88. Lp00122
89. Nc00423
90. Nsc-759560
91. {2-[4-(2-butyl-1-benzofuran-3-carbonyl)-2,6-diiodophenoxy]ethyl}diethylamine Hydrochloride
92. Amiodarone Hydrochloride [mart.]
93. Amiodarone Hydrochloride [vandf]
94. Amiodarone Hydrochloride [usp-rs]
95. Amiodarone Hydrochloride [who-dd]
96. Ncgc00093613-01
97. Ncgc00093613-02
98. Ncgc00093613-03
99. Ncgc00093613-04
100. Ncgc00254295-01
101. Ncgc00260807-01
102. Ba139263
103. Hy-14188
104. Methanone, (2-butyl-3-benzofuranyl)(4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl)-, Hydrochloride
105. Methanone,5-diiodophenyl]-, Hydrochloride
106. Eu-0100122
107. Ft-0622300
108. Sw196856-4
109. Amiodarone Hydrochloride [orange Book]
110. A 8423
111. Amiodarone Hydrochloride [ep Monograph]
112. Amiodarone Hydrochloride [usp Monograph]
113. D00636
114. D86460
115. 774a824
116. Q-200626
117. Sr-01000003087-2
118. Sr-01000003087-8
119. Wln: T56 Boj C4 Dvr Ci Ei Do2n2&2 &gh
120. Q27271976
121. Z2210825534
122. Amiodarone Hydrochloride 1.0 Mg/ml In Methanol (as Free Base)
123. (2-butylbenzofuran-3-yl)(4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl)methanonehydrochloride
124. 2-butyl-3-benzofuranyl 4-[(2-diethylamino)ethoxy] 3,5-diiodophenyl Ketone, Hydrochloride
125. Ketone, 2-butyl-3-benzofuranyl 4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl, Hydrochloride
126. (2-butyl-1-benzofuran-3-yl)-[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone;hydron;chloride
127. (2-butyl-1-benzofuran-3-yl){4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl}methanone Hydrochloride (1:1)
128. (2-butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone Hydrochloride
129. Amiodarone Hydrochloride Solution, 1.0 Mg/ml In Methanol (as Free Base), Ampule Of 1 Ml, Certified Reference Material
130. Methanone, (2-butyl-3-benzofuranyl)(4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl)- Hydrochloride
131. Methanone, (2-butyl-3-benzofuranyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]-, Hydrochloride (1:1)
| Molecular Weight | 681.8 g/mol |
|---|---|
| Molecular Formula | C25H30ClI2NO3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 11 |
| Exact Mass | 681.00037 g/mol |
| Monoisotopic Mass | 681.00037 g/mol |
| Topological Polar Surface Area | 42.7 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 547 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 6 | |
|---|---|
| Drug Name | Amiodarone hydrochloride |
| Drug Label | Amiodarone hydrochloride is a member of a class of antiarrhythmic drugs with predominantly Class III (Vaughan Williams classification) effects, available for oral administration as pink, scored tablets containing 200 mg of amiodarone hydrochloride... |
| Active Ingredient | Amiodarone hydrochloride |
| Dosage Form | Injectable; Tablet |
| Route | Injection; Oral |
| Strength | 300mg; 50mg/ml; 200mg; 100mg; 400mg |
| Market Status | Prescription |
| Company | Wockhardt; Teva Pharms; Fresenius Kabi Usa; Hospira; Gland Pharma; Mylan Institutional; Apotex; Hikma Farmaceutica; Taro; Zydus Pharms Usa; Sandoz; Mylan; Murty Pharms; Akorn; Barr |
| 2 of 6 | |
|---|---|
| Drug Name | Nexterone |
| PubMed Health | Amiodarone |
| Drug Classes | Antiarrhythmic, Group III |
| Drug Label | NEXTERONE contains amiodarone HCl (C25H29I2NO3HCl), a class III antiarrhythmic drug. Amiodarone HCl is (2-butyl-3-benzo-furanyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone hydrochloride.Amiodarone HCl has the following structural formul... |
| Active Ingredient | Amiodarone hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 360mg/200ml (1.8mg/ml); 150mg/100ml (1.5mg/ml); 50mg/ml |
| Market Status | Prescription |
| Company | Baxter Hlthcare |
| 3 of 6 | |
|---|---|
| Drug Name | Pacerone |
| Drug Label | Pacerone (amiodarone hydrochloride) Tablets are a member of a class of antiarrhythmic drugs with predominantly Class III (Vaughan Williams classification) effects, available for oral administration as light yellow, scored tablets. Each tablet fo... |
| Active Ingredient | Amiodarone hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 100mg |
| Market Status | Prescription |
| Company | Upsher Smith |
| 4 of 6 | |
|---|---|
| Drug Name | Amiodarone hydrochloride |
| Drug Label | Amiodarone hydrochloride is a member of a class of antiarrhythmic drugs with predominantly Class III (Vaughan Williams classification) effects, available for oral administration as pink, scored tablets containing 200 mg of amiodarone hydrochloride... |
| Active Ingredient | Amiodarone hydrochloride |
| Dosage Form | Injectable; Tablet |
| Route | Injection; Oral |
| Strength | 300mg; 50mg/ml; 200mg; 100mg; 400mg |
| Market Status | Prescription |
| Company | Wockhardt; Teva Pharms; Fresenius Kabi Usa; Hospira; Gland Pharma; Mylan Institutional; Apotex; Hikma Farmaceutica; Taro; Zydus Pharms Usa; Sandoz; Mylan; Murty Pharms; Akorn; Barr |
| 5 of 6 | |
|---|---|
| Drug Name | Nexterone |
| PubMed Health | Amiodarone |
| Drug Classes | Antiarrhythmic, Group III |
| Drug Label | NEXTERONE contains amiodarone HCl (C25H29I2NO3HCl), a class III antiarrhythmic drug. Amiodarone HCl is (2-butyl-3-benzo-furanyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone hydrochloride.Amiodarone HCl has the following structural formul... |
| Active Ingredient | Amiodarone hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 360mg/200ml (1.8mg/ml); 150mg/100ml (1.5mg/ml); 50mg/ml |
| Market Status | Prescription |
| Company | Baxter Hlthcare |
| 6 of 6 | |
|---|---|
| Drug Name | Pacerone |
| Drug Label | Pacerone (amiodarone hydrochloride) Tablets are a member of a class of antiarrhythmic drugs with predominantly Class III (Vaughan Williams classification) effects, available for oral administration as light yellow, scored tablets. Each tablet fo... |
| Active Ingredient | Amiodarone hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 200mg; 100mg |
| Market Status | Prescription |
| Company | Upsher Smith |
Anti-Arrhythmia Agents; Enzyme Inhibitors; Vasodilator Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Anti-arrhythmic class III
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 85
Amiodarone in the oral dosage form is indicated only in the treatment of recurrent hemodynamically unstable ventricular tachycardia and recurrent ventricular fibrillation unresponsive to documented adequate doses of other available antiarrythmic medications or when alternative agents cannot be tolerated. In patients from whom the oral form of amiodarone is indicated, but who are unable to take oral medication, the intravenous form may be used.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 95
Amiodarone is used to suppress and prevent recurrence of supraventricular arrythmias refractory to conventional treatment, especially when associated with Wolff-Parkinson-White (W-P-W) syndrome, including paroxysmal atrial fibrillation, atrial fibrillation, atrial flutter, ectopic atrial tachycardia, and paroxysmal supraventricular tachycardia from both atriovantricular (AV) nodal re-entrant and AV re-entrant tachycardia in patients with W-P-W syndrome.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 95
Amiodarone has been used in a limited number of patients for the management of chronic stable angina pectoris.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1511
Pulmonary toxicity, which is potentially fatal, is the most severe adverse effect associated with oral amiodarone therapy. Amiodarone induced pulmonary toxicity may result from pulmonary interstitial pneumonitis (or alveolitis) or from hypersensitivity pneumonitis. Clinically apparent interstitial pneumonitis (or alveolitis), hypersensitivity pneumonitis, and pulmonary fibrosis have occurred in up to 10-17% of patients with ventricular arrhythmias receiving amiodarone hydrochloride therapy at oral dosages of about 400 mg daily, and an abnormal diffusion capacity without symptoms occurs in a much higher percentage of patients. ... Amiodarone induced pulmonary toxicity has been fatal in about 10% of cases. Rarely, amiodarone has been associated with exacerbation of bronchial asthma, possibly because of its antiadrenergic effects in at least one patient receiving aminodarone. Hemoptysis was reported.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1513
Amiodarone induced peripheral neuropathy ... is usually symmetrical and involves all four limbs; the neurologic deficit is usually more marked in the lower limbs than in the upper limbs. Signs and symptoms may include distal sensory loss, sensory ataxia, loss of vibratory sensation, paresthesia, and/or decreased tendon reflexes. Proximal muscle weakness may also be present. Nerve biopsies in patients with amiodarone induced peripheral neuropathy have demonstrated complete loss of a large myelinated fibers, marked reduction of small myelinated and unmyelinated axons, and evidence of lysosomal inclusion bodies within Schwann cells. Nerve conduction studies have demonstrated normal or reduced nerve conduction velocities.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1514
Amiodarone induced hypothyroidism has been reported in about 2-4% of patients receiving oral drug therapy in most clinical studies, although this effect may occur more frequently. Limited data suggest that hypothyroidism may be more likely to occur in females and in patients with a prior history of thyroid dysfunction.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1514
Arrhythmogenic effects associated with amiodarone have occurred in approximately 2-5% of patients and have included progression of ventricular tachycardia to ventricular fibrillation, sustained ventricular tachycardia, increased resistance to cardioversion, atrial fibrillation, nodal arrhythmia, and atypical ventricular tachycardia (torsade de pointes). Transient exacerbation of preexisting cardiac arrhythmias with subsequent control during continued therapy has also been reported. ... Acceleration of ventricular rate was reported in a patient receiving IV amiodarone for the treatment of atrial fibrillation associated with Wolff-Parkinson-White syndrome.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1514
For more Drug Warnings (Complete) data for AMIODARONE HYDROCHLORIDE (28 total), please visit the HSDB record page.
Cytochrome P-450 CYP1A2 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP1A2. (See all compounds classified as Cytochrome P-450 CYP1A2 Inhibitors.)
Cytochrome P-450 CYP3A Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inhibitors.)
Sodium Channel Blockers
A class of drugs that act by inhibition of sodium influx through cell membranes. Blockade of sodium channels slows the rate and amplitude of initial rapid depolarization, reduces cell excitability, and reduces conduction velocity. (See all compounds classified as Sodium Channel Blockers.)
Potassium Channel Blockers
A class of drugs that act by inhibition of potassium efflux through cell membranes. Blockade of potassium channels prolongs the duration of ACTION POTENTIALS. They are used as ANTI-ARRHYTHMIA AGENTS and VASODILATOR AGENTS. (See all compounds classified as Potassium Channel Blockers.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Cytochrome P-450 CYP2C9 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP2C9. (See all compounds classified as Cytochrome P-450 CYP2C9 Inhibitors.)
Cytochrome P-450 CYP2D6 Inhibitors
Drugs and compounds which inhibit or antagonize the biosynthesis or actions of CYTOCHROME P-450 CYP2D6. (See all compounds classified as Cytochrome P-450 CYP2D6 Inhibitors.)
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
Plasma concentration of amiodarone appear to decline in at least a biphasic manner, although more complex, multicompartmental pharmacokinetics have been described. Following a single IV dose in healthy adults, the half-life of the drug in the terminal elimination phase has been reported to average 25 days (range 9-47 days). The elimination half-life of the major metabolite, N-desethylamiodarone, is equal to or longer than that of the parent drug. Following single dose admin of amiodarone in a limited number of healthy individuals, amiodarone exhibits multicompartmental pharmacokinetics; the mean apparent terminal plasma elimination half-life of amiodarone and N-desethylamiodarone were 58 (range: 15-142) and 36 (range: 14-75) days, respectively. The half-life of amiodarone appears to be substantially more prolonged following multiple rather than single doses. It has been suggested that differences in reported elimination half-lives may result in part from misinterpretation of slow distribution phases as elimination phases following IV administration of the drug. Following chronic oral administration of amiodarone hydrochloride in patients with cardiac arrhythmias (200-600 mg daily for 2-52 months), the drug appears to be eliminated in a biphasic manner with an initial elimination half-life of about 2.5-10 days, which is followed by a terminal elimination half-life averaging 53 days (range: 26-107 days), with most patients exhibiting a terminal elimination half-life in the range of 40-55 days. The elimination half-life of the major metabolite, N-desethylamiodarone, averages 57-61 days (range 20-118 days) following long-term oral administration of amiodarone. The elimination profile of amiodarone may reflect an initial elimination of the drug from well-perfused tissues followed by prolonged elimination from poorly perfused tissues such as adipose tissue.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1522
Following iv administration of amiodarone in healthy individuals, total plasma clearance of the drug averages approximately 1.9 ml/min/kg (range: 1.4-2.5 ml/min/kg). Although not clearly established, total apparent plasma clearance of the drug appears to decrease with time. Clinical experience suggests that clearance of amiodarone may be more rapid in pediatric patients; however, further studies are needed to fully determine the effects of age on clearance of the drug. Factors of age, gender, or renal or hepatic disease appear to have no effect on the disposition of amiodarone or its major metabolite, N-desethylamiodarone.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1522
Amiodarone hydrochloride is slowly and variably absorbed from the GI tract following oral administration. The absolute bioavailability of commercially available amiodarone hydrochloride tablets averages approximately 50%, but varies considerably, ranging from 22-86%.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1521
Following oral administration, peak plasma amiodarone concentrations usually occur within 3-7 hours (range: 2-12 hours). Following oral administration of a single 400 mg dose of amiodarone hydrochloride in fasting, healthy adults, peak plasma amiodarone concentration of approximately 0.15-0.7 ug/ml are attained. Within the oral dosage range of 100-600 mg daily, steady state plasma concentrations of the drug are approximately proportional to dosage, increasing by an average of 0.5 ug/ml per 100 mg increment in dosage; however, there is considerable interindividual variation in plasma concentrations attained with a given dosage. Following continuous oral administration of the drug in the absence of an initial loading dose regimen, steady state plasma amiodarone concentrations would not be attained for at least 1 month and generally not for up to 5 months or longer. Following chronic oral administration of amiodarone, plasma concentrations of N-desethylamiodarone, the major metabolite of the drug, are approximately 0.5-2 times those of unchanged drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1521
For more Absorption, Distribution and Excretion (Complete) data for AMIODARONE HYDROCHLORIDE (11 total), please visit the HSDB record page.
The drug appears to be extensively metabolized, probably in the liver and possibly in the intestinal lumen and/or GI mucosa, to at least one major metabolite. The major metabolite, N-desethylamiodarone, is formed by N-deethylation. Although not clearly established, limited data in animals indicate that the desethyl metabolite may possess some antiarrhythmic activity. ... A minor metabolite of amiodarone, di-N-desethylamiodarone, has been identified in animals following chronic administration of the drug. Amiodarone and N-desethylamiodarone may undergo deiodination to form deiodoamiodarone and deiodo-N-desethylamiodarone, respectively; iodine (in the form of iodide); and possibly other iodine containing metabolites. It is not known whether deiodinated metabolites are pharmacologically active.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 835
Amiodarone undergoes hepatic metabolism by cytochrome P450 3A4 to desethyl-amiodarone.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 956
Following a single IV dose in healthy adults, the half-life of the drug in the terminal elimination phase has been reported to average 25 days (range 9-47 days). The elimination half-life of the major metabolite, N-desethylamiodarone, is equal to or longer than that of the parent drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1522
Following single dose admin of amiodarone in a limited number of healthy individuals, amiodarone exhibits multicompartmental pharmacokinetics; the mean apparent terminal plasma elimination half-life of amiodarone and N-desethylamiodarone were 58 (range: 15-142) and 36 (range: 14-75) days, respectively.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1522
Following chronic oral administration of amiodarone hydrochloride in patients with cardiac arrhythmias (200-600 mg daily for 2-52 months), the drug appears to be eliminated in a biphasic manner with an initial elimination half-life of about 2.5-10 days, which is followed by a terminal elimination half-life averaging 53 days (range: 26-107 days), with most patients exhibiting a terminal elimination half-life in the range of 40-55 days.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1522
The elimination half-life of the major metabolite, N-desethylamiodarone, averages 57-61 days (range 20-118 days) following long-term oral administration of amiodarone.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1522
Amiodarone prolongs the action potential duration of myocardial cells without altering the resting membrane potential. Consequently the drug alters repolarization (QT prolongation) without affecting spontaneous (phase 4) depolarization. Since neither class I nor class II antiarrhythmic drugs possess both these properties, amiodarone is classified as a class III antiarrhythmic agent. The effect of amiodarone on thyroid metabolism remains unclear but probably involves an intracellular rather than a central or peripheral action.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning. 2nd ed. Baltimore, MD: Williams and Wilkins, 1997., p. 529
Although several investigators have suggested that the myocardial effects observed during chronic amiodarone therapy are comparable to those associated with hypothyroidism and may be related to competitive inhibition of sodium-potassium-activated adenosine triphosphatase (Na+ -K+ -ATPase) activity, other data suggest that amiodarone's effects on thyroid function contribute minimally, if at all, to the overall electrophysiologic effects of the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1520
The drug also appears to inhibit transmembrane influx of extracellular sodium ions via fast sodium channels, as indicated by a decrease in the maximal rate of depolarization of phase O of the action potential. Like class I antiarrhythmic agents, amiodarone is believed to combine with fast sodium channels in their inactive state and thereby inhibit recovery after repolarization in a time- and voltage-dependent manner which is associated with subsequent dissociation of the drug from the sodium channels. Amiodarone appears to have little affinity for activated fast sodium channels.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 2003. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2003 (Plus Supplements)., p. 1520
Amiodarone has mild negative inotropic effect that is more prominent with intravenous than with oral administration but that usually does not depress left ventrical function. Amiodarone causes coronary and peripheral vasodialation and, therefore, decrease peripheral vascular resistance but only causes hypotension in large doses.
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 23rd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2003. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 89

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
62
PharmaCompass offers a list of Amiodarone API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Amiodarone manufacturer or Amiodarone supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Amiodarone manufacturer or Amiodarone supplier.
PharmaCompass also assists you with knowing the Amiodarone API Price utilized in the formulation of products. Amiodarone API Price is not always fixed or binding as the Amiodarone Price is obtained through a variety of data sources. The Amiodarone Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Amiodarone manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Amiodarone, including repackagers and relabelers. The FDA regulates Amiodarone manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Amiodarone API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Amiodarone manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Amiodarone supplier is an individual or a company that provides Amiodarone active pharmaceutical ingredient (API) or Amiodarone finished formulations upon request. The Amiodarone suppliers may include Amiodarone API manufacturers, exporters, distributors and traders.
click here to find a list of Amiodarone suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Amiodarone DMF (Drug Master File) is a document detailing the whole manufacturing process of Amiodarone active pharmaceutical ingredient (API) in detail. Different forms of Amiodarone DMFs exist exist since differing nations have different regulations, such as Amiodarone USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Amiodarone DMF submitted to regulatory agencies in the US is known as a USDMF. Amiodarone USDMF includes data on Amiodarone's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Amiodarone USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Amiodarone suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Amiodarone Drug Master File in Japan (Amiodarone JDMF) empowers Amiodarone API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Amiodarone JDMF during the approval evaluation for pharmaceutical products. At the time of Amiodarone JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Amiodarone suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Amiodarone Drug Master File in Korea (Amiodarone KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Amiodarone. The MFDS reviews the Amiodarone KDMF as part of the drug registration process and uses the information provided in the Amiodarone KDMF to evaluate the safety and efficacy of the drug.
After submitting a Amiodarone KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Amiodarone API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Amiodarone suppliers with KDMF on PharmaCompass.
A Amiodarone CEP of the European Pharmacopoeia monograph is often referred to as a Amiodarone Certificate of Suitability (COS). The purpose of a Amiodarone CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Amiodarone EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Amiodarone to their clients by showing that a Amiodarone CEP has been issued for it. The manufacturer submits a Amiodarone CEP (COS) as part of the market authorization procedure, and it takes on the role of a Amiodarone CEP holder for the record. Additionally, the data presented in the Amiodarone CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Amiodarone DMF.
A Amiodarone CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Amiodarone CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Amiodarone suppliers with CEP (COS) on PharmaCompass.
A Amiodarone written confirmation (Amiodarone WC) is an official document issued by a regulatory agency to a Amiodarone manufacturer, verifying that the manufacturing facility of a Amiodarone active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Amiodarone APIs or Amiodarone finished pharmaceutical products to another nation, regulatory agencies frequently require a Amiodarone WC (written confirmation) as part of the regulatory process.
click here to find a list of Amiodarone suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Amiodarone as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Amiodarone API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Amiodarone as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Amiodarone and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Amiodarone NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Amiodarone suppliers with NDC on PharmaCompass.
Amiodarone Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Amiodarone GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Amiodarone GMP manufacturer or Amiodarone GMP API supplier for your needs.
A Amiodarone CoA (Certificate of Analysis) is a formal document that attests to Amiodarone's compliance with Amiodarone specifications and serves as a tool for batch-level quality control.
Amiodarone CoA mostly includes findings from lab analyses of a specific batch. For each Amiodarone CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Amiodarone may be tested according to a variety of international standards, such as European Pharmacopoeia (Amiodarone EP), Amiodarone JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Amiodarone USP).
