
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
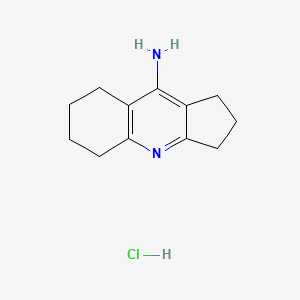

1. Amiridine
2. Axamon
3. Ipidacrine
4. Neuromidin
5. Nik 247
6. Nik247
1. Ipidacrine Hydrochloride
2. 90043-86-0
3. 2,3,5,6,7,8-hexahydro-1h-cyclopenta[b]quinolin-9-amine Hydrochloride
4. Ipidacrine Monohydrochloride
5. Ipidacrine (hydrochloride)
6. Nik 247;amiridin; Amiridine
7. 1h-cyclopenta(b)quinoline, 2,3,5,6,7,8-hexahydro-9-amino-, Monohydrochloride
8. 1h,2h,3h,5h,6h,7h,8h-cyclopenta[b]quinolin-9-amine Hydrochloride
9. 107d610372
10. Nik 247
11. 9-amino-2,3,5,6,7,8-hexahydro-1h-cyclopenta[b]quinoline Hydrochloride
12. 9-amino-2,3,5,6,7,8-hexahydro-1h-cyclopenta(b)quinoline Hydrochloride
13. Unii-107d610372
14. 2,3,5,6,7,8-hexahydro-9-amino-1h-cyclopenta(b)quinoline Hydrochloride
15. 9-amino-2,3,5,6,7,8-hexahydro-1h-cyclopenta(b)quinoline Monohydrochloride
16. Schembl1032513
17. Dtxsid00238003
18. Ipidacrine Hydrochloride [mi]
19. Akos025397206
20. Ipidacrine Hydrochloride [who-dd]
21. Ft-0691362
22. Ec-000.2106
23. En300-74223
24. A843421
25. Q27251128
26. 2,3,5,6,7,8-hexahydro-1h-cyclopenta[b]quinolin-9-amine Hcl
27. 2,3,5,6,7,8-hexahydro-1h-cyclopenta[b]quinolin-9-amine;hydrochloride
28. 1h-cyclopenta(b)quinolin-9-amine, 2,3,5,6,7,8-hexahydro-, Hydrochloride (1:1)
29. 1h-cyclopenta(b)quinolin-9-amine, 2,3,5,6,7,8-hexahydro-, Monohydrochloride
| Molecular Weight | 224.73 g/mol |
|---|---|
| Molecular Formula | C12H17ClN2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 224.1080262 g/mol |
| Monoisotopic Mass | 224.1080262 g/mol |
| Topological Polar Surface Area | 38.9 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 216 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?