
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
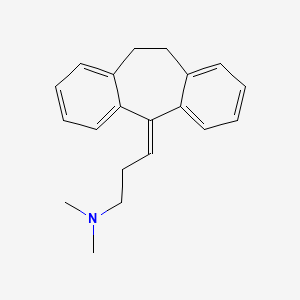

1. Amineurin
2. Amitrip
3. Amitriptylin Beta
4. Amitriptylin Desitin
5. Amitriptylin Neuraxpharm
6. Amitriptylin Rph
7. Amitriptylin-neuraxpharm
8. Amitriptyline Hydrochloride
9. Amitrol
10. Anapsique
11. Apo Amitriptyline
12. Apo-amitriptyline
13. Damilen
14. Desitin, Amitriptylin
15. Domical
16. Elavil
17. Endep
18. Laroxyl
19. Lentizol
20. Novoprotect
21. Rph, Amitriptylin
22. Saroten
23. Sarotex
24. Syneudon
25. Triptafen
26. Tryptanol
27. Tryptine
28. Tryptizol
1. 50-48-6
2. Amitriptylin
3. Damitriptyline
4. Seroten
5. Triptanol
6. Flavyl
7. Proheptadiene
8. Elavil
9. Damilen
10. Lantron
11. Adepress
12. Triptisol
13. Tryptanol
14. Adepril
15. Amitriprolidine
16. Amitryptiline
17. Triptilin
18. Laroxyl
19. Redomex
20. Damilan
21. Ro 4-1575
22. Sarotex
23. Mk 230
24. 3-(10,11-dihydro-5h-dibenzo[a,d][7]annulen-5-ylidene)-n,n-dimethylpropan-1-amine
25. 5-(3-dimethylaminopropylidene)-10,11-dihydro-5h-dibenzo(a,d)cycloheptene
26. 3-(10,11-dihydro-5h-dibenzo(a,d)cyclohepten-5-ylidene)-n,n-dimethyl-1-propanamine
27. 10,11-dihydro-5-(gamma-dimethylaminopropylidene)-5h-dibenzo(a,d)cycloheptene
28. Amitriptyline (inn)
29. Hsdb 3007
30. Chembl629
31. 5-(3'-dimethylaminopropylidene)-dibenzo-(a,d)(1,4)-cycloheptadiene
32. 3-(10,11-dihydro-5h-dibenzo(a,d)cyclohepten-5-yliden)-n,n-dimethylpropylamin
33. 5h-dibenzo(a,d)cycloheptene-delta5,gamma-propylamine, 10,11-dihydro-n,n-dimethyl-
34. Chebi:2666
35. Amytriptylin
36. 5-(gamma-dimethylaminopropylidene)-5h-dibenzo[a,d][1,4]cycloheptadiene
37. Limbitrol
38. 5-(3-dimethylaminopropylidene)-10,11-dihydro-5h-dibenzo(a,d)cycloheptatriene
39. 1-propanamine, 3-(10,11-dihydro-5h-dibenzo(a,d)cyclohepten-5-ylidene)-n,n-dimethyl-
40. 1-propanamine, 3-(10,11-dihydro-5h-dibenzo[a,d]cyclohepten-5-ylidene)-n,n-dimethyl-
41. 10,11-dihydro-n,n-dimethyl-5h-dibenzo(a,d)heptalene-delta(sup 5),gamma-propylamine
42. 1806d8d52k
43. Amitriptilina
44. Amitriptylinum
45. Amitryptyline
46. Amytriptiline
47. Laroxil
48. Elanil
49. Amitriptylin [german]
50. Amitriptilina [italian]
51. 10,11-dihydro-n,n-dimethyl-5h-dibenzo(a,d)heptalene-delta(5),gamma-propylamine
52. Amitriptyline [inn]
53. 3-(10,11-dihydro-5h-dibenzo[a,d]cyclohepten-5-ylidene)-n,n-dimethylpropan-1-amine
54. Amitriptyline [inn:ban]
55. Amitriptylinum [inn-latin]
56. Amitriptilina [inn-spanish]
57. 5-(3'-dimethylaminopropylidene)-dibenzo-[a,d][1,4]-cycloheptadiene
58. 10,11-dihydro-5-(gamma-dimethylaminopropylidene)-5h-dibenzo[a,d]cycloheptene
59. 5-(gamma-dimethylaminopropylidene)-5h-dibenzo(a,d)(1,4)cycloheptadiene
60. 3-(10,11-dihydro-5h-dibenzo[a,d]cyclohepten-5-ylidene)-n,n-dimethyl-1-propanamine
61. 5h-dibenzo[a,d]cycloheptene-.delta.5,.gamma.-propylamine, 10,11-dihydro-n,n-dimethyl-
62. Laroxyl (tn)
63. Ccris 9174
64. Nsc169910
65. N 750
66. Ncgc00015095-09
67. Cas-549-18-8
68. Einecs 200-041-6
69. Brn 2217885
70. Unii-1806d8d52k
71. 5-(gamma-dimethylaminopropylidine)-5h-dibenzo(a,d)(1,4)cycloheptadiene
72. 5-(gamma-dimethylaminopropylidene)-10,11-dihydro-5h-dibenzo(a,d)cycloheptene
73. Domical (salt/mix)
74. Laroxyl (salt/mix)
75. Lentizol (salt/mix)
76. Vanatrip (salt/mix)
77. Triptizol (salt/mix)
78. Spectrum_000044
79. Prestwick0_000074
80. Prestwick1_000074
81. Prestwick2_000074
82. Prestwick3_000074
83. Spectrum2_000101
84. Spectrum3_000298
85. Spectrum4_000146
86. Spectrum5_000806
87. Lopac-a-8404
88. Amitriptyline [mi]
89. Ec 200-041-6
90. Schembl7824
91. 3-(10,11-dihydro-5h-dibenzo[a,d][7]annulen-5-ylidene)-n,n-dimethyl-1-propanamine
92. Lopac0_000112
93. Oprea1_479304
94. Amitriptyline [vandf]
95. Bspbio_000287
96. Bspbio_001836
97. Gtpl200
98. Kbiogr_000592
99. Kbiogr_002261
100. Kbioss_000424
101. Kbioss_002262
102. Amitriptyline [mart.]
103. Bidd:gt0115
104. Divk1c_000766
105. Spbio_000082
106. Spbio_002208
107. Amitriptyline [who-dd]
108. Bpbio1_000317
109. Dtxsid7022594
110. Kbio1_000766
111. Kbio2_000424
112. Kbio2_002261
113. Kbio2_002992
114. Kbio2_004829
115. Kbio2_005560
116. Kbio2_007397
117. Kbio3_001336
118. Kbio3_002741
119. Cmap_000001
120. Ninds_000766
121. Zinc968257
122. 5-(3-dimethylaminopropylidene)dibenzo[a,d][1,4]-cycloheptadiene
123. Bcp09083
124. Amitriptyline [usp Impurity]
125. Bdbm50020712
126. Pdsp1_001564
127. Pdsp2_001548
128. S5947
129. Stk525215
130. 5-(3-dimethylaminopropylidene)-5h-dibenzo[a,d]-10,11-dihydrocycloheptene
131. 5-(gamma-dimethylaminopropylidene)-5h-dibenzo[a,d]10,11-dihydrocycloheptene
132. Akos000512694
133. Ccg-204207
134. Db00321
135. Sdccgsbi-0050100.p005
136. 5h-dibenzo(a,d)cycloheptene-delta(sup 5),gamma-propylamine, 10,11-dihydro-n,n-dimethyl-
137. Idi1_000766
138. Mrf-0000533
139. Ncgc00015095-01
140. Ncgc00015095-02
141. Ncgc00015095-03
142. Ncgc00015095-04
143. Ncgc00015095-05
144. Ncgc00015095-06
145. Ncgc00015095-07
146. Ncgc00015095-08
147. Ncgc00015095-10
148. Ncgc00015095-11
149. Ncgc00015095-12
150. Ncgc00015095-14
151. Ncgc00015095-26
152. Ncgc00024433-04
153. Ncgc00183047-01
154. Dimethyl({3-[(2z)-tricyclo[9.4.0.0^{3,8}]pentadeca-1(11),3(8),4,6,12,14-hexaen-2-ylidene]propyl})amine
155. N,n-dimethyl-3-(2-tricyclo[9.4.0.03,8]pentadeca-1(15),3,5,7,11,13-hexaenylidene)propan-1-amine
156. Sbi-0050100.p004
157. Ab00514631
158. Amitriptylinemk-230, N-750, Ro41575
159. Bb 0305430
160. Ft-0653242
161. C06824
162. D07448
163. Q58397
164. Ab00053417-12
165. Ab00053417_13
166. Ab00053417_14
167. 412a072
168. L001041
169. W-109252
170. Brd-k53737926-003-05-4
171. Brd-k53737926-003-14-6
172. Nortriptyline Hydrochloride Impurity F [ep Impurity]
173. 5-(.gamma.-dimethylaminopropylidene)-5h-dibenzo[a,d][1,4]cycloheptadiene
174. 5-(.gamma.-dimethylaminopropylidine)-5h-dibenzo(a,d)(1,4)cycloheptadiene
175. 10,11-dihydro-5-(.gamma.-dimethylaminopropylidene)-5h-dibenzo(a,d)cycloheptene
176. 10,11-dihydro-n,n-dimethl-5h-dibenzo[a,d]cycloheptene-(delta(5, Gamma))-propylamine
177. 10,11-dihydro-n,n-dimethyl-5h-dibenzo(a,d)heptalene-.delta.5,.gamma.-propylamine
178. 10,11-dihydro-n,n-dimethyl-5h-dibenzo(a,d)heptalene-delta5,gamma-propylamine
179. 3-(5,6-dihydrodibenzo[[?],[?]][7]annulen-11-ylidene)-n,n-dimethyl-propan-1-amine
180. 5-(.gamma.-dimethylaminopropylidene)-10,11-dihydro-5h-dibenzo(a,d)cycloheptene
181. 5-(.gamma.-dimethylaminopropylidene)-5h-dibenzo(a,d)-10,11-dihydrocycloheptene
182. 3-(10,11-dihydro-5h-dibenzo-[a,d]cyclohepten-5-ylidene)-n,n-dimethyl-1-propanamine, Hydrochloride
183. 5h-dibenzo(a,d)cycloheptene-.delta.(sup 5),.gamma.-propylamine, 10,11-dihydro-n,n-dimethyl-
184. 5h-dibenzo[a,d]cycloheptene-delta5,gamma-propylamine, 10,11-dihydro-n,n-dimethyl- (6ci,8ci) 5-(gamma-dimethylaminopropylidene)-10,11-dihydro-5h-dibenzo[a,d]cycloheptene
185. Dimethyl({3-[(2e)-tricyclo[9.4.0.0^{3,8}]pentadeca-1(15),3,5,7,11,13-hexaen-2-ylidene]propyl})amine
| Molecular Weight | 277.4 g/mol |
|---|---|
| Molecular Formula | C20H23N |
| XLogP3 | 5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 3 |
| Exact Mass | 277.183049738 g/mol |
| Monoisotopic Mass | 277.183049738 g/mol |
| Topological Polar Surface Area | 3.2 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 331 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Adrenergic Uptake Inhibitors; Analgesics, Non-Narcotic; Antidepressive Agents, Tricyclic
National Library of Medicine's Medical Subject Headings. Amitriptyline. Online file (MeSH, 2016). Available from, as of January 20, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Amitriptyline is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of March 17, 2016: https://clinicaltrials.gov/ct2/results?term=AMITRIPTYLINE&Search=Search
For the relief of symptoms of depression. Endogenous depression is more likely to be alleviated than are other depressive states. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Amitriptyline Hydrochloride Tablet, Film Coated (Updated: November 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1e6d2c80-fbc8-444e-bdd3-6a91fe1b95bd
Tricyclic antidepressants have been used for the treatment of attention deficit hyperactivity disorder (ADHD). /Tricyclic antidepressant; NOT included in US product label/
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2392
For more Therapeutic Uses (Complete) data for AMITRIPTYLINE (13 total), please visit the HSDB record page.
/BOXED WARNING/ Suicidality and Antidepressant Drugs: Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of amitriptyline hydrochloride tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Amitriptyline hydrochloride is not approved for use in pediatric patients.
NIH; DailyMed. Current Medication Information for Amitriptyline Hydrochloride Tablet, Film Coated (Updated: November 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1e6d2c80-fbc8-444e-bdd3-6a91fe1b95bd
A syndrome resembling neuroleptic malignant syndrome (NMS) has been very rarely reported after starting or increasing the dose of amitriptyline hydrochloride, with and without concomitant medications known to cause NMS. Symptoms have included muscle rigidity, fever, mental status changes, diaphoresis, tachycardia, and tremor.
NIH; DailyMed. Current Medication Information for Amitriptyline Hydrochloride Tablet, Film Coated (Updated: November 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1e6d2c80-fbc8-444e-bdd3-6a91fe1b95bd
Very rare cases of serotonin syndrome (SS) have been reported with amitriptyline hydrochloride in combination with other drugs that have a recognized association with SS.
NIH; DailyMed. Current Medication Information for Amitriptyline Hydrochloride Tablet, Film Coated (Updated: November 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1e6d2c80-fbc8-444e-bdd3-6a91fe1b95bd
Amitriptyline hydrochloride ... should be used with caution in patients with a history of seizures and, because of its atropine-like action, in patients with a history of urinary retention, angle-closure glaucoma or increased intraocular pressure. In patients with angle-closure glaucoma, even average doses may precipitate an attack.
NIH; DailyMed. Current Medication Information for Amitriptyline Hydrochloride Tablet, Film Coated (Updated: November 2015). Available from, as of January 20, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1e6d2c80-fbc8-444e-bdd3-6a91fe1b95bd
For more Drug Warnings (Complete) data for AMITRIPTYLINE (39 total), please visit the HSDB record page.
This drug in indicated for the following conditions: Major depressive disorder in adults Management of neuropathic pain in adults Prophylactic treatment of chronic tension-type headache (CTTH) in adults Prophylactic treatment of migraine in adults Treatment of nocturnal enuresis in children aged 6 years and above when organic pathology, including spina bifida and related disorders, have been excluded and no response has been achieved to all other non-drug and drug treatments, including antispasmodics and vasopressin-related products. This product should only be prescribed by a healthcare professional with expertise in the management of persistent enuresis Off-label uses: irritable bowel syndrome, sleep disorders, diabetic neuropathy, agitation, fibromyalgia, and insomnia
FDA Label
**Effects in pain and depression** Amitriptyline is a tricyclic antidepressant and an analgesic. It has anticholinergic and sedative properties. Clinical studies have shown that oral amitriptyline achieves, at a minimum, good to moderate response in up to 2/3 of patients diagnosed with post-herpetic neuralgia and 3/4 of patients diagnosed with diabetic neuropathic pain, and neurogenic pain syndromes that are frequently unresponsive to narcotic analgesics. Amitriptyline has also shown efficacy in diverse groups of patients with chronic non-malignant pain. There have also been some studies showing efficacy in managing fibromyalgia (an off-label use of this drug),. **Cardiovascular and Anticholinergic Effects** Amitriptyline has strong anticholinergic properties and may cause ECG changes and quinidine-like effects on the heart. Amitriptyline may inhibit ion channels, which are necessary for cardiac repolarization (hERG channels), in the upper micromolar range of therapeutic plasma concentrations. Therefore, amitriptyline may increase the risk for cardiac arrhythmia. Orthostatic hypotension and tachycardia can be a problem in elderly patients receiving this drug at normal doses for depression. There is evidence in the literature that these effects may occur, rarely, at the lower dosages utilized in the treatment of pain. As with any other tricyclic antidepressant agent, increased glucose levels can occur with amitriptyline. **Effects on seizure threshold** This drug also decreases the convulsive threshold and causes alterations in EEG and sleep patterns.
Analgesics, Non-Narcotic
A subclass of analgesic agents that typically do not bind to OPIOID RECEPTORS and are not addictive. Many non-narcotic analgesics are offered as NONPRESCRIPTION DRUGS. (See all compounds classified as Analgesics, Non-Narcotic.)
Adrenergic Uptake Inhibitors
Drugs that block the transport of adrenergic transmitters into axon terminals or into storage vesicles within terminals. The tricyclic antidepressants (ANTIDEPRESSIVE AGENTS, TRICYCLIC) and amphetamines are among the therapeutically important drugs that may act via inhibition of adrenergic transport. Many of these drugs also block transport of serotonin. (See all compounds classified as Adrenergic Uptake Inhibitors.)
Antidepressive Agents, Tricyclic
Substances that contain a fused three-ring moiety and are used in the treatment of depression. These drugs block the uptake of norepinephrine and serotonin into axon terminals and may block some subtypes of serotonin, adrenergic, and histamine receptors. However, the mechanism of their antidepressant effects is not clear because the therapeutic effects usually take weeks to develop and may reflect compensatory changes in the central nervous system. (See all compounds classified as Antidepressive Agents, Tricyclic.)
N06AA09
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AA - Non-selective monoamine reuptake inhibitors
N06AA09 - Amitriptyline
Absorption
Rapidly absorbed following oral administration (bioavailability is 30-60% due to first pass metabolism). Peak plasma concentrations are reached 2-12 hours after oral or intramuscular administration. Steady-state plasma concentrations vary greatly and this variation may be due to genetic differences.
Route of Elimination
Amitriptyline and its metabolites are mainly excreted in the urine. Virtually the entire dose is excreted as glucuronide or sulfate conjugate of metabolites, with approximately 2% of unchanged drug appearing in the urine. 25-50% of a single orally administered dose is excreted in urine as inactive metabolites within 24 hours. Small amounts are excreted in feces via biliary elimination.
Volume of Distribution
The apparent volume of distribution (Vd) estimated after intravenous administration is 1221 L280 L; range 769-1702 L (163 L/kg). It is found widely distributed throughout the body. Amitriptyline and the main metabolite _nortriptyline_ pass across the placental barrier and small amounts are present in breast milk.
Clearance
The mean systemic clearance (Cls) is 39.24 10.18 L/h (range: 24.53-53.73 L/h). No clear effect of older age on the pharmacokinetics of amitriptyline has been determined, although it is possible that clearance may be decreased.
This study reports the pharmacokinetics of oral amitriptyline and its active metabolite nortriptyline in Greyhound dogs. Five healthy Greyhound dogs were enrolled in a randomized crossover design. A single oral dose of amitriptyline hydrochloride (actual mean dose 8.1 per kg) was administered to fasted or fed dogs. Blood samples were collected at predetermined times from 0 to 24 hr after administration, and plasma drug concentrations were measured by liquid chromatography with mass spectrometry. Noncompartmental pharmacokinetic analyses were performed. Two dogs in the fasted group vomited following amitriptyline administration and were excluded from analysis. The range of amitriptyline CMAX for the remaining fasted dogs (n = 3) was 22.8-64.5 ng/mL compared to 30.6-127 ng/mL for the fed dogs (n = 5). The range of the amitriptyline AUCINF for the three fasted dogs was 167-720 hr ng/mL compared to 287-1146 hr ng/mL for fed dogs. The relative bioavailability of amitriptyline in fasted dogs compared to fed dogs was 69-91% (n = 3). The exposure of the active metabolite nortriptyline was correlated to amitriptyline exposure (R(2) = 0.84). Due to pharmacokinetic variability and the small number of dogs completing this study, further studies are needed assessing the impact of feeding on oral amitriptyline pharmacokinetics. Amitriptyline may be more likely to cause vomiting in fasted dogs.
PMID:25989225 Norkus C et al; J Vet Pharmacol Ther. 2015 Dec;38(6):619-22 (2015)
The inability of a 30-yr-old female with graft-versus-host disease to absorb oral doses of amitriptyline was reported. Only trace plasma levels of the drug were determined following 4 wk therapy with 50 mg daily doses. Additional therapy with 75 mg daily doses for 10 days failed to incr the antidepressant's plasma levels.
Freed E et al; Med J Aust 140 (Apr 14): 509-510 (1984)
An experimental rat model was used to study postmortem redistribution of amitriptyline. Two hr after a sc injection with 20 mg of amitriptyline, the rats (n=40) were anesthetized and blood samples were drawn from the femoral vein and the heart. The rats were then sacrificed by CO2 and left at room temp for either 0.1, 1, 2, 5, 24, 48, or 96 hr. Postmortem blood samples from the heart and the inferior vena cava, and tissue samples from the lungs, heart, liver, right kidney, thigh muscle, the wall of the abdominal vena cava and brain were analyzed by HPLC. A significant incr was observed within 2 hr postmortem in heart blood and later also in blood from the inferior vena cava. At 96 hr postmortem the concn incr was 4.4 + or - 0.5-fold (p < 0.01) and 3.0 + or - 1.1-fold (p < 0.05) as compared to the antemortem values observed in heart blood and blood from the inferior vena cava, respectively (mean + or - SEM). In the lungs there was a fall in the concn of AMI from 148 + or - 16.7 umol/kg at 0.1 hr to 49.1 + or - 7.8 umol/kg at 96 hr postmortem (p < 0.01). In the vessel wall of the abdominal vena cava there was also a significant fall in drug concn, while in heart muscle and liver an incr in drug concn was observed. In animals where the lungs were removed agonally (n = 7), the drug concn in heart blood had incr significantly less at 2 hr postmortem.
PMID:8157229 Hilberg T et al; Forensic Sci Int 64 (1): 47-55 (1994)
The percutaneous absorption of amitriptyline, nortriptyline, imipramine, and desipramine as their hydrochloride salts in vivo was demonstrated without use of a vehicle using the hairless (hr-1/hr-1) mouse as an experimental model for human skin. After topical application of 2 mg of each compound in distilled water, followed by rapid evaporation of the water, concn were measured in heart, lung, brain, liver, and blood in 1-, 2-, 4-, and 6-hr study groups. Lung consistently demonstrated the highest concn for all four compounds while heart and liver had the lowest. Concn in heart remained essentially constant for all compounds during the 6-hour study period. The concn in solid tissues were much lower than those commonly seen in man after overdose, whereas the concn in blood resembled low therapeutic to toxic concn in humans. Percutaneous absorption may provide a feasible route of admin for the tricyclic antidepressants which may lead to improved compliance with fewer GI side effects. /Amitriptyline hydrochloride/
PMID:2395341 Bailey DN; J Anal Toxicol 14 (4): 217-8 (1990)
For more Absorption, Distribution and Excretion (Complete) data for AMITRIPTYLINE (17 total), please visit the HSDB record page.
In vitro, the metabolism of amitriptyline occurs mainly by demethylation (CYP2C19, CYP3A4) as well as hydroxylation (CYP2D6) followed by conjugation with glucuronic acid. Other isozymes involved in amitriptyline metabolism are CYP1A2 and CYP2C9. The metabolism of this drug is subject to genetic polymorphisms. The main active metabolite is the secondary amine, _nortriptyline_. Nortriptyline is a stronger inhibitor of noradrenaline than of serotonin uptake, while amitriptyline inhibits the uptake of noradrenaline and serotonin with equal efficacy. Other metabolites such as _cis-_ and _trans-10-hydroxyamitriptyline_ and _cis-_ and _trans-10-hydroxynortriptyline_ have the same pharmacologic profile as nortriptyline but are significantly weaker. _Demethylnortriptyline_ and amitriptyline N oxide are only present in plasma in negligible amounts; the latter is mostly inactive.
A method for the determination of amitriptyline and some of its metabolites in serum on a reversed phase system consisting of C-8 bonded phase material as the stationary phase and water-methanol-dichloromethane-propylamine as the mobile phase by liquid chromatography with UV detection at 254 nm is described. ... Serum levels of amitriptyline and its 4 main metabolites (nortriptyline, desmethylnortriptyline, trans-10-hydroxyamitriptyline and trans-10-hydroxynortriptyline) in a patient receiving 150 mg of oral amitriptyline daily are reported.
Kraak JC, Bijster P; J Chromatogr Biomed 143 (Sep 1): 499-512 (1977)
Amitriptyline is metabolized via the same pathways as are other tricyclic antidepressants; nortriptyline, its N-monodemethylated metabolite, is pharmacologically active.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2399
To investigate the metabolism of amitriptyline and debrisoquine ... 8 healthy Chinese volunteers received a single oral dose of 100 mg amitriptyline and the ratios between the area under the curve (AUC) of amitriptyline and its 3 metabolites were evaluated. Results indicated that large interindividual differences in AUC were observed. In addition, hydroxylation of amitriptyline and debrisoquine may be regulated by similar enzymatic processes.
Zhang XH et al; Acta Pharm Sinica (Yao Hsueh Hsueh Pao) 28 (Feb): 85-91 (1993)
Biotransformation of amitriptyline to its demethylated product nortriptyline was studied in vitro with human liver microsomes from four different donors, preselected to reflect a range of metabolic rates. Reaction velocity versus substrate concn was consistent with a sigmoid Vmax model. Vmax varied from 0.42 to 3.42 nmol/mg/min, Km from 33 to 89 uM amitriptyline. Ketoconazole was a highly potent inhibitor of N-demethylation, with a mean Ki value of 0.11 + or - 0.013 uM ... whereas quinidine (up to 50 uM), a CYP2D6 inhibitor, and alpha-naphthoflavone (up to 5 uM), a CYP1A2 inhibitor only at low concn, showed no effect. All selective serotonin reuptake inhibitors tested had an inhibitory effect on the formation of nortriptyline, with mean Ki values of 4.37 (+ or - 3.38) uM for sertraline, 5.46 (+ or - 1.95) uM for desmethylsertraline, 9.22 (+ or - 3.69) uM for fluvoxamine, 12.26 (+ or - 5.67) uM for norfluoxetine, 15.76 (+ or - 5.50) uM for paroxetine, and 43.55 (+ or - 18.28) uM for fluoxetine. A polyclonal rabbit antibody against rat liver CYP3A1, in antibody/microsomal protein rations varying from 1:1 to 10:1, inhibited N-demethylation of amitriptyline to an asymptotic max of 60%.
PMID:7473143 Schmider J et al; J Pharmacol Exp Ther 275 (2): 592-7 (1995)
For more Metabolism/Metabolites (Complete) data for AMITRIPTYLINE (8 total), please visit the HSDB record page.
Amitriptyline has known human metabolites that include E-10-hydroxyamitriptyline and nortriptyline.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The elimination half-life (t12 ) amitriptyline after peroral administration is about 25 hours (24.65 6.31 hours; range 16.49-40.36 hours).
The plasma half-life of amitriptyline ranges from 10 to 50 hours.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2399
The toxicokinetics of amitriptyline were studied in nine patients admitted to hospital in Matthew-Lawson Coma Scale grade III-IV after an estimated ingestion of 1-5 g amitriptyline. ... The T1/2 alpha and T1/2 beta for amitriptyline were 1.5 - 3.1 and 15 - 43 hr respectively. ...
PMID:1588668 Hulten BA et al; J Toxicol Clin Toxicol 30 (2): 181-201 (1992)
... The purpose of this pilot study was to describe the individual and population pharmacokinetic parameters of amitriptyline after a single oral dose at 1.5 mg/kg, 4.5 mg/kg, and 9 mg/kg in healthy African grey parrots ( Psittacus erithacus , n = 3) and cockatoos (Cacatua species, n = 3). Three birds received an initial 1.5 mg/kg oral dose, and blood samples were collected for 24 hours at fixed time intervals. Serum concentrations of amitriptyline and its metabolites were determined by polarized immunofluorescence. After determining the initial parameters and a 14-day washout period, 2 African grey parrots and 1 cockatoo received a single oral dose at 4.5 mg/kg, and 3 cockatoos and 1 African grey parrot received a single oral dose at 9 mg/kg. ... Elimination half-life varied from 1.6 to 91.2 hours. ...
PMID:26771316 Visser M et al; J Avian Med Surg 29 (4): 275-81 (2015)
The mechanism of action of this drug is not fully elucidated. It is suggested that amitriptyline inhibits the membrane pump mechanism responsible for the re-uptake of transmitter amines, such as norepinephrine and serotonin, thereby increasing their concentration at the synaptic clefts of the brain,. These amines are important in regulating mood. The monoamine hypothesis in depression, one of the oldest hypotheses, postulates that deficiencies of serotonin (5-HT) and/or norepinephrine (NE) neurotransmission in the brain lead to depressive effects. This drug counteracts these mechanisms, and this may be the mechanism of amitriptyline in improving depressive symptoms. Whether its analgesic effects are related to its mood-altering activities or attributable to a different, less obvious pharmacological action (or a combination of both) is unknown.
Acute and chronic effects of the antidepressant drugs tranylcypromine, a monoamine oxidase inhibitor, and amitriptyline, a monoamine uptake inhibitor, were studied on beta-adrenergic receptor function in mouse astrocytes in primary cultures. In clinically relevant concentrations, acute administration of either antidepressant drug had a direct inhibitory effect on the binding of the beta-adrenergic ligand dihydroalprenolol and on the isoproterenol-induced accumulation of cyclic AMP. However, in the absence of isoproterenol, these drugs enhanced the formation of cyclic AMP in the astrocytes. Chronic exposure to amitriptyline or tranylcypromine led to a decrease in isoproterenol-induced accumulation of cyclic AMP, and the time course for the development of this phenomenon was similar to that reported for whole brain in vivo. These findings suggest that these antidepressant drugs act as a partial agonists at beta-adrenergic receptors on astrocytes, and that the down-regulation of beta-adrenergic activity that occurs in vivo after chronic administration of antidepressant drugs may, to a large extent, take place in astrocytes and may result from the partial beta-agonist nature of the drugs.
PMID:6302296 Hertz L, Richardson JS; J Neurosci Res. 1983;9(2):173-82 (1983)
Astrocytes play important roles in guiding the construction of the nervous system, controlling extracellular ions and neurotransmitters, and regulating CNS synaptogenesis. Egr-1 is a transcription factor involved in neuronal differentiation and astrocyte cell proliferation. In this study, we investigated whether the tricyclic antidepressant (TCA) amitriptyline induces Egr-1 expression in astrocytes using rat C6 glioma cells as a model. We found that amitriptyline increased the expression of Egr-1 in a dose- and time-dependent manner. The amitriptyline-induced Egr-1 expression was mediated through serum response elements (SREs) in the Egr-1 promoter. SREs were activated by the Ets-domain transcription factor Elk-1 through the ERK and JNK mitogen-activated protein (MAP) kinase pathways. The inhibition of the ERK and JNK MAP kinase signals attenuated amitriptyline-induced transactivation of Gal4-Elk-1 and Egr-1 promoter activity. Our findings suggest that the induction of Egr-1 expression in astrocytes may be required to attain the therapeutic effects of antidepressant drugs.
PMID:17590509 Chung EY et al; Neurosci Lett 422 (1): 43-8 (2007)
Antidepressants such as serotonin-noradrenaline reuptake inhibitors (SNRIs) and tricyclic antidepressants (TCAs) are frequently used for the management of neuropathic pain. Noradrenaline (NA) and serotonin (5-HT) increase in the spinal cord by reuptake inhibition is considered to be main mechanism of the therapeutic effect of antidepressants in neuropathic pain. In the present study, we examined the analgesic effects of duloxetine (SNRI) and amitriptyline (TCA) in a rat model of neuropathic pain induced by spinal nerve ligation (SNL). Intraperitoneal administration of duloxetine and amitriptyline dose-dependently (3,10 and 30 mg/kg) suppressed hyperalgesia induced by SNL. In vivo microdialysis in the lumbar spinal dorsal horn revealed that NA and 5-HT concentrations increased after intraperitoneal administration of duloxetine and amitriptyline (10 mg/kg, respectively). We further determined NA and 5-HT contents in homogenized samples from the ipsilateral dorsal spinal cord after SNL. Although the NA content in SNL rats 2 weeks after ligation was higher than that in SNL rats 4 weeks after ligation, the analgesic efficacy of duloxetine and amitriptyline was similar between two groups. The present study suggests that NA/5-HT increase in the spinal cord is crucial in the antihyperalgesic effect of duloxetine and amitriptyline. The plastic change of the descending noradrenergic system does not obviously affect the analgesic efficacy of duloxetine and amitriptyline.
PMID:26135544 Hoshino H et al; Neurosci Lett 602: 62-7 (2015)
Recent studies show that neuronal and glial plasticity are important for the therapeutic action of antidepressants. Here, we demonstrated that amitriptyline, a tricyclic antidepressant, significantly increased GDNF mRNA and GDNF release in C6 cells. Furthermore, different classes of antidepressants increased GDNF release, but non-antidepressant psychotropic drugs did not. The amitriptyline-induced GDNF release was completely inhibited by U0126, a mitogen-activated protein kinase (MAPK)-extracellular signal-regulated kinase (ERK) kinase (MEK) inhibitor, but was not inhibited by H-89, a protein kinase A inhibitor or calphostin C, a protein kinase C inhibitor. These results suggest that the amitriptyline-induced GDNF release may be regulated through a MEK/MAPK pathway. Next, we examined the effects of monoamines on GDNF release, because antidepressants are known to increase monoamines. 5-HT increased GDNF mRNA and GDNF release, but noradrenaline and dopamine did not. The 5-HT-induced GDNF release was partially, but significantly, blocked by ketanserin, a 5-HT2A receptor antagonist. The 5-HT-induced GDNF release was completely inhibited by U0126, but was not inhibited by H-89 or calphostin C. These results suggest that the 5-HT-induced GDNF release was mediated through a MEK/MAPK pathway and, at least, 5-HT2A receptors. GDNF, as well as other neurotrophic factors, may contribute to explain the therapeutic action of antidepressants and suggest a novel strategy of pharmacological intervention.
PMID:15796067 Hisaoka K et al; Nihon Shinkei Seishin Yakurigaku Zasshi 25 (1): 25-31 (2005)
For more Mechanism of Action (Complete) data for AMITRIPTYLINE (13 total), please visit the HSDB record page.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18174
Submission : 2005-03-02
Status : Inactive
Type : II

USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
USDMF, CEP/COS, JDMF, EU-WC, NDC, KDMF, VMF, Others
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4253
Submission : 1981-08-05
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF






 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18174
Submission : 2005-03-02
Status : Inactive
Type : II

USDMF
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 4253
Submission : 1981-08-05
Status : Inactive
Type : II

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Trepiline
Dosage Form : TAB
Dosage Strength : 25mg
Packaging : 100X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Trepiline
Dosage Form : TAB
Dosage Strength : 25mg
Packaging : 500X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Trepiline 10
Dosage Form : TAB
Dosage Strength : 10mg
Packaging : 100X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Trepiline 10
Dosage Form : TAB
Dosage Strength : 10mg
Packaging : 500X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Veikirin 10
Dosage Form : TAB
Dosage Strength : 10mg
Packaging : 100X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Veikirin 25
Dosage Form : TAB
Dosage Strength : 25mg
Packaging : 100X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : Egypt
Brand Name :
Dosage Form : TABLET
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : Egypt

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic CTD, BE
Registration Country : India
Brand Name :
Dosage Form : TABLET
Dosage Strength : 25MG
Packaging :
Approval Date :
Application Number : 85966
Regulatory Info : Generic CTD, BE
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic CTD, BE
Registration Country : India
Brand Name :
Dosage Form : TABLET
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number : 40218
Regulatory Info : Generic CTD, BE
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : TABLET
Dosage Strength : 10MG
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

Analyte : Amitriptyline
Method : LSMS/MS
LLOQ : 25
Units : ng/mL
Details : Human Plasma

Analyte : Amitriptyline
Method : LSMS/MS
LLOQ : 6
Units : ng/mL
Details : Human Plasma

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
47
PharmaCompass offers a list of Amitriptyline API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Amitriptyline manufacturer or Amitriptyline supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Amitriptyline manufacturer or Amitriptyline supplier.
PharmaCompass also assists you with knowing the Amitriptyline API Price utilized in the formulation of products. Amitriptyline API Price is not always fixed or binding as the Amitriptyline Price is obtained through a variety of data sources. The Amitriptyline Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Amitriptyline manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Amitriptyline, including repackagers and relabelers. The FDA regulates Amitriptyline manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Amitriptyline API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Amitriptyline manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Amitriptyline supplier is an individual or a company that provides Amitriptyline active pharmaceutical ingredient (API) or Amitriptyline finished formulations upon request. The Amitriptyline suppliers may include Amitriptyline API manufacturers, exporters, distributors and traders.
click here to find a list of Amitriptyline suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Amitriptyline DMF (Drug Master File) is a document detailing the whole manufacturing process of Amitriptyline active pharmaceutical ingredient (API) in detail. Different forms of Amitriptyline DMFs exist exist since differing nations have different regulations, such as Amitriptyline USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Amitriptyline DMF submitted to regulatory agencies in the US is known as a USDMF. Amitriptyline USDMF includes data on Amitriptyline's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Amitriptyline USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Amitriptyline suppliers with USDMF on PharmaCompass.
Amitriptyline Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Amitriptyline GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Amitriptyline GMP manufacturer or Amitriptyline GMP API supplier for your needs.
A Amitriptyline CoA (Certificate of Analysis) is a formal document that attests to Amitriptyline's compliance with Amitriptyline specifications and serves as a tool for batch-level quality control.
Amitriptyline CoA mostly includes findings from lab analyses of a specific batch. For each Amitriptyline CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Amitriptyline may be tested according to a variety of international standards, such as European Pharmacopoeia (Amitriptyline EP), Amitriptyline JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Amitriptyline USP).

