
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
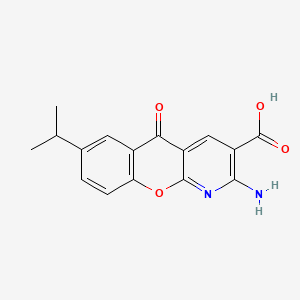

1. 2-amino-7-isopropyl-5-oxo-5h-(1)benzopyrano(2,3b)pyridine-3-carboxylic Acid
2. Aa 673
3. Aa-673
4. Amoxanox
5. Aphthasol
6. Chx 3673
1. 68302-57-8
2. Amoxanox
3. Aphthasol
4. 2-amino-7-isopropyl-5-oxo-5h-chromeno[2,3-b]pyridine-3-carboxylic Acid
5. Amlenanox
6. Elics
7. Amlexanoxum [latin]
8. Aa-673
9. Amlexanoxo [spanish]
10. Amlexanoxo
11. Amlexanoxum
12. Chx 3673
13. Aphtheal
14. Chx-3673
15. 2-amino-5-oxo-7-propan-2-ylchromeno[2,3-b]pyridine-3-carboxylic Acid
16. 2-amino-5-oxo-7-(propan-2-yl)-5h-chromeno[2,3-b]pyridine-3-carboxylic Acid
17. 2-amino-7-isopropyl-5-oxo-5h-(1)benzopyrano(2,3-b)pyridine-3-carboxylic Acid
18. Aa673;amoxanox;chx3673
19. Brl1c2459k
20. Chebi:31205
21. 2-amino-7-(1-methylethyl)-5-oxo-5h-chromeno[2,3-b]pyridine-3-carboxylic Acid
22. Ncgc00167472-01
23. Amlexanox [usan:inn:jan]
24. Dsstox_cid_2595
25. 2-amino-7-isopropyl-5-oxo-5h-chromeno-[2,3-b]pyridine-3-carboxylic Acid
26. Aa 673
27. 5h-(1)benzopyrano(2,3-b)pyridine-3-carboxylic Acid, 2-amino-7-(1-methylethyl)-5-oxo-
28. Dsstox_rid_76651
29. Dsstox_gsid_22595
30. Aptheal
31. Apthera
32. Orarinse
33. Oradisc A
34. Aphthasol (tn)
35. Anw
36. Smr000466352
37. Cas-68302-57-8
38. Ccris 2686
39. Solfa (tn)
40. Brn 0556384
41. Unii-brl1c2459k
42. 4wbo
43. 5h-[1]benzopyrano[2,3-b]pyridine-3-carboxylic Acid, 2-amino-7-(1-methylethyl)-5-oxo-
44. Amlexanox [usan:inn:ban:jan]
45. Amlexanox- Bio-x
46. Mfcd00864790
47. 2-amino-7-(1-methylethyl)-5-oxo-5h-[1]benzopyrano[2,3-b]pyridine-3-carboxylic Acid
48. Aa-673(amlexanox)
49. Amlexanox [inn]
50. Amlexanox [jan]
51. Amlexanox [mi]
52. Amlexanox [usan]
53. Amlexanox [vandf]
54. Amlexanox [mart.]
55. Amlexanox [who-dd]
56. Chembl1096
57. Schembl29642
58. Zinc928
59. Mls000759466
60. Mls001424059
61. Mls006010129
62. Bidd:gt0709
63. Us10214536, Amlexanox
64. Gtpl7113
65. Amlexanox (jp17/usan/inn)
66. Amlexanox [orange Book]
67. Amlexanox, >=98% (hplc)
68. Dtxsid2022595
69. Chx3673
70. Aa673
71. Bdbm357857
72. Hms2051f13
73. Hms2235m08
74. Hms3393f13
75. Hms3715b14
76. Bcp28222
77. Hy-b0713
78. Tox21_112476
79. 2-amino-7-isopropyl-5-oxochromeno[2,3-b]pyridine-3-carboxylic Acid
80. S3648
81. 2-amino-7-isopropyl-5-oxo-5h-chromeno[2,3-b]pyridine-3-carboxylicacid
82. Akos015900498
83. Tox21_112476_1
84. Ac-1192
85. Ccg-100953
86. Db01025
87. Ds-1396
88. Nc00203
89. Ncgc00167472-02
90. Ncgc00167472-03
91. Ncgc00167472-14
92. Ba164161
93. Ft-0641176
94. D01828
95. Ab00639947-06
96. Ab00639947_08
97. 302a578
98. A836094
99. L001037
100. Q695611
101. Amlexanox, United States Pharmacopeia (usp) Reference Standard
102. 2-amino-7-isopropyl-5-oxo-5h-[1]benzopyrano[2,3-b] Pyridine-3-carboxylic Acid
103. 2-amino-7-isopropyl-5-oxo-chromeno[2,3-b]pyridine-3-carboxylic Acid;amlexanox
104. 2-amino-7-(1-methylethyl)-5-oxo-5h-[1]benzopyrano[2,3-b]pyridine-3-carboxylic Acid Amoxanox Aa-673 Chx-3673 Aphthasol Elics Solfa
105. 5h-(1)benzopyrano(2,3-.beta.)pyridine-3-carboxylic Acid, 2-amino-7-(1-methylethyl)-5-oxo-
| Molecular Weight | 298.29 g/mol |
|---|---|
| Molecular Formula | C16H14N2O4 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 298.09535693 g/mol |
| Monoisotopic Mass | 298.09535693 g/mol |
| Topological Polar Surface Area | 103 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 467 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used as a paste in the mouth to treat aphthous ulcers (canker sores).
FDA Label
Amlexanox is a mucoadhesive oral paste which has been clinically proven to abort the onset, accelerate healing and resolve the pain of aphthous ulcers (canker sores). It decreases the time ulcers take to heal. Because amlexanox decreases the healing time, it also decreases the pain you feel. Recent studies have also shown that the majority of ulcers can be prevented by application of the paste during the prodromal (pre-ulcerative) phase of the disease. Recurrent Aphthous Ulcers (RAU) also known as Recurrent Aphthous Stomatitis (RAS) is recognized as the most common oral mucosal disease known to man. Estimates suggest that 20% - 25% of the general population suffer at least one incidence of aphthous ulcers each year. Amlexanox is also being investigated for its anti-allergenic and anti-inflammatory properties.
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
A - Alimentary tract and metabolism
A01 - Stomatological preparations
A01A - Stomatological preparations
A01AD - Other agents for local oral treatment
A01AD07 - Amlexanox
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03D - Other systemic drugs for obstructive airway diseases
R03DX - Other systemic drugs for obstructive airway diseases
R03DX01 - Amlexanox
Absorption
No significant absorption directly through the active ulcer. Most of the systemic absorption is via the gastrointestinal tract.
Metabolized to hydroxylated and conjugated metabolites.
Elimination half-life is 3.5 ± 1.1 hours.
As a benzopyrano-bipyridine carboxylic acid derivative, amlexanox has anti-inflammatory and antiallergic properties. It inhibits chemical mediatory release of the slow-reacting substance of anaphylaxis (SRS-A) and may have antagonistic effects on interleukin-3. When cells are under stress, they release an inactive form of human fibroblast growth factor 1 (FGF-1), a potent mitogen (entity that causes mitosis). Amlexanox binds to FGF1, increasing its conformational stability, sterically blocking Cu(2+) induced oxidation which normally leads to activation of FGF-1.
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
73
PharmaCompass offers a list of Amlexanox API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Amlexanox manufacturer or Amlexanox supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Amlexanox manufacturer or Amlexanox supplier.
PharmaCompass also assists you with knowing the Amlexanox API Price utilized in the formulation of products. Amlexanox API Price is not always fixed or binding as the Amlexanox Price is obtained through a variety of data sources. The Amlexanox Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Amlexanox manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Amlexanox, including repackagers and relabelers. The FDA regulates Amlexanox manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Amlexanox API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Amlexanox manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Amlexanox supplier is an individual or a company that provides Amlexanox active pharmaceutical ingredient (API) or Amlexanox finished formulations upon request. The Amlexanox suppliers may include Amlexanox API manufacturers, exporters, distributors and traders.
click here to find a list of Amlexanox suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Amlexanox as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Amlexanox API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Amlexanox as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Amlexanox and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Amlexanox NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Amlexanox suppliers with NDC on PharmaCompass.
Amlexanox Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Amlexanox GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Amlexanox GMP manufacturer or Amlexanox GMP API supplier for your needs.
A Amlexanox CoA (Certificate of Analysis) is a formal document that attests to Amlexanox's compliance with Amlexanox specifications and serves as a tool for batch-level quality control.
Amlexanox CoA mostly includes findings from lab analyses of a specific batch. For each Amlexanox CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Amlexanox may be tested according to a variety of international standards, such as European Pharmacopoeia (Amlexanox EP), Amlexanox JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Amlexanox USP).

