
Synopsis
0
CEP/COS
0
EU WC
0
VMF
0
FDA Orange Book

0
Canada

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
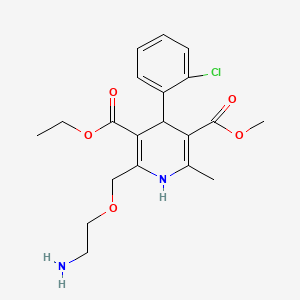

1. Amlodipine Besylate
2. Amlodipine Maleate
3. Amlodipine Maleate (1:1)
4. Amlodipine, (+-)-isomer
5. Amlodipine, (+-)-isomer, Maleate (1:1)
6. Amlodipine, (r)-isomer
7. Amlodipine, (s)-isomer, Maleate (1:1)
8. Amlodis
9. Amlor
10. Astudal
11. Istin
12. Norvasc
1. 88150-42-9
2. Norvasc
3. Amlodipine Base
4. Amlodipino
5. Amlodipinum
6. Caduet
7. Istin
8. Amlodipine Free Base
9. Amvaz
10. Amlodis
11. Chebi:2668
12. 3-ethyl 5-methyl 2-((2-aminoethoxy)methyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate
13. Norvasc (tn)
14. Amlocard
15. Coroval
16. Lipinox
17. 3-ethyl 5-methyl 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate
18. Uk-4834011
19. Amlodipinum [latin]
20. Hgp0904
21. Amlodipino [spanish]
22. 88150-42-9 (free Base)
23. Hgp-0904
24. Amdepin
25. Amdipin
26. Amlodin
27. Pelmec
28. Ckd-330 Component Amlodipine
29. Mfcd00864687
30. 1j444qc288
31. 3,5-pyridinedicarboxylic Acid, 2-((2-aminoethoxy)methyl)-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-, 3-ethyl 5-methyl Ester
32. 3-o-ethyl 5-o-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate
33. Amlodipine [inn:ban]
34. Racemic Amlodipine
35. (rs)-3-ethyl 5-methyl 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate
36. 3,5-pyridinedicarboxylic Acid, 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-, 3-ethyl 5-methyl Ester
37. 3-ethyl 5-methyl 2-{[(2-aminoethyl)oxy]methyl}-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate
38. Sr-05000001461
39. Ncgc00165957-04
40. Amlodipine 100 Microg/ml In Acetonitrile
41. Unii-1j444qc288
42. Amlodipine D4
43. Hsdb 7079
44. 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine
45. 2-[2-aminoethoxymethyl]-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine
46. Amlodipine (norvasc)
47. Amlodipine [mi]
48. Amlodipine (usp/inn)
49. Amlodipine [inn]
50. Spectrum2_000486
51. Spectrum3_001004
52. Spectrum4_001132
53. Spectrum5_001550
54. Amlodipine [vandf]
55. Ec 425-820-1
56. Amlodipine [mart.]
57. Amlodipine [who-dd]
58. Chembl1491
59. Schembl26478
60. Bspbio_002727
61. Kbiogr_001643
62. Mls001401409
63. Amlodipine [ema Epar]
64. Bidd:gt0810
65. Spbio_000351
66. Gtpl6981
67. Amlodipine [orange Book]
68. Chembl3211346
69. Chembl3304444
70. Dtxsid7022596
71. Kbio3_001947
72. Copalia (amlodipine + Valsartan)
73. Morphine Sulfate Pentahydrate Usp
74. Bcpp000403
75. Hms2052n03
76. Hms2089h07
77. Hms2231k08
78. Hms3370g17
79. Hms3394n03
80. Hms3651i04
81. Hms3713c10
82. Albb-027270
83. Bcp02420
84. Hy-b0317
85. Bdbm50088383
86. S1905
87. Stl356053
88. Akos015843475
89. Ac-4535
90. Bcp9000295
91. Ccg-101157
92. Ccg-220414
93. Db00381
94. Nc00407
95. Ncgc00165957-01
96. Ncgc00165957-02
97. Ncgc00165957-03
98. Ncgc00165957-05
99. Ncgc00165957-07
100. 3-ethyl 5-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate
101. 3-ethyl-5-methyl (+-)-2-((2-aminoethoxy)methyl)-4-(2-chlorphenyl)-1,4-dihydro-6-methyl-3,5-pyridindicarboxylat
102. 3-ethyl-5-methyl (+-)-2-((2-aminoethoxymethyl)-4-(o-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate
103. 3-ethyl-5-methyl (+-)-2-(2-aminoethoxymethyl)-4-(o-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate
104. As-13747
105. Ba164164
106. Cpd000469198
107. O3-ethyl O5-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate
108. Smr000469198
109. Sy104813
110. Uk-48340
111. Ft-0602653
112. Ft-0657130
113. Ft-0662111
114. Ft-0662112
115. Sw220228-1
116. 50a429
117. C06825
118. D07450
119. Ab01209618-01
120. Ab01274726-01
121. Ab01274726-02
122. Ab01274726_03
123. Ab01274726_04
124. Ab01274726_05
125. A842481
126. Q411347
127. Katerzia® (amlodipine Oral Suspension, 1 Mg/ml)
128. Sr-05000001461-3
129. Sr-05000001461-4
130. Sr-05000001461-5
131. Brd-a22032524-074-02-4
132. Brd-a22032524-074-03-2
133. Brd-a22032524-074-04-0
134. 2-(2-aminoethoxy)methyl-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine
135. 2-[(2-aminoethoxy)-methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5- Pyridinedicarboxylic Acid 3-ethyl 5-methyl Ester Benzene Sulfonate
136. 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylic Acid 3-ethyl 5-methylester
137. 2-[2-aminoethoxymethyl]-3-ethoxycarbonyl-4-(2-chlorophenyl)-5-methoxycarbonyl-6-methyl-1,4-dihydropyridine
138. 3,5-pyridinedicarboxylic Acid, 2-((2-aminoethoxy)methyl)-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-, 3-ethyl 5-methyl Ester, (+/-)-
139. 3,5-pyridinedicarboxylic Acid, 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-, 3-ethyl 5-methyl Ester, Benzene Sulfonate
140. 3-ethyl 5-methyl (+/-)-2-((2-aminoethoxy)methyl)-4-(o-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate
141. 3-ethyl 5-methyl Ester 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylic Acid
142. 3-ethyl 5-methylester, (+/-)-2-[(2-aminoethoxy)methyl]-4-(o-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate
143. 3-ethyl-5-methyl (.+/-.)-2-[(2-aminoethoxy)methyl]-4-(o-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate
144. 3-ethyl5-methyl2-((2-aminoethoxy)methyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate
145. 3-o-ethyl 5-o-methyl 4-(2-chlorophenyl)-1-deuterio-2-[2-(dideuterioamino)ethoxymethyl]-6-methyl-4h-pyridine-3,5-dicarboxylate
146. O3-ethyl O5-methyl 2-(2-azanylethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate
| Molecular Weight | 408.9 g/mol |
|---|---|
| Molecular Formula | C20H25ClN2O5 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 10 |
| Exact Mass | 408.1451996 g/mol |
| Monoisotopic Mass | 408.1451996 g/mol |
| Topological Polar Surface Area | 99.9 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 647 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Caduet |
| PubMed Health | Amlodipine/Atorvastatin (By mouth) |
| Drug Classes | Calcium Channel Blocker/HMG-COA Reductase Inhibitor Combination |
| Active Ingredient | atorvastatin calcium; Amlodipine besylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 40mg base; eq 80mg base; eq 5mg base; eq 2.5mg base; eq 20mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Pfizer |
| 2 of 4 | |
|---|---|
| Drug Name | Norvasc |
| PubMed Health | Amlodipine (By mouth) |
| Drug Classes | Antianginal, Antihypertensive, Cardiovascular Agent |
| Drug Label | NORVASC is the besylate salt of amlodipine, a long-acting calcium channel blocker.Amlodipine besylate is chemically described as 3-Ethyl-5-methyl ()-2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate, monobe... |
| Active Ingredient | Amlodipine besylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 5mg base; eq 2.5mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Pfizer |
| 3 of 4 | |
|---|---|
| Drug Name | Caduet |
| PubMed Health | Amlodipine/Atorvastatin (By mouth) |
| Drug Classes | Calcium Channel Blocker/HMG-COA Reductase Inhibitor Combination |
| Active Ingredient | atorvastatin calcium; Amlodipine besylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 40mg base; eq 80mg base; eq 5mg base; eq 2.5mg base; eq 20mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Pfizer |
| 4 of 4 | |
|---|---|
| Drug Name | Norvasc |
| PubMed Health | Amlodipine (By mouth) |
| Drug Classes | Antianginal, Antihypertensive, Cardiovascular Agent |
| Drug Label | NORVASC is the besylate salt of amlodipine, a long-acting calcium channel blocker.Amlodipine besylate is chemically described as 3-Ethyl-5-methyl ()-2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate, monobe... |
| Active Ingredient | Amlodipine besylate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 5mg base; eq 2.5mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Pfizer |
Antihypertensive Agents; Calcium Channel Blockers; Vasodilator Agents
National Library of Medicine's Medical Subject Headings. Amlodipine. Online file (MeSH, 2016). Available from, as of October 28, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
Norvasc is indicated for the treatment of hypertension, to lower blood pressure. ... Norvasc may be used alone or in combination with other antihypertensive agents. /Included in US product label/
NIH; DailyMed. Current Medication Information for Norvasc (Amlodipine Besylate) Tablet (Updated: April 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=abd6a2ca-40c2-485c-bc53-db1c652505ed
Norvasc is indicated for the symptomatic treatment of chronic stable angina. Norvasc may be used alone or in combination with other antianginal agents. /Included in US product label/
NIH; DailyMed. Current Medication Information for Norvasc (Amlodipine Besylate) Tablet (Updated: April 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=abd6a2ca-40c2-485c-bc53-db1c652505ed
Norvasc is indicated for the treatment of confirmed or suspected vasospastic angina. Norvasc may be used as monotherapy or in combination with other antianginal agents. /Included in US product label/
NIH; DailyMed. Current Medication Information for Norvasc (Amlodipine Besylate) Tablet (Updated: April 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=abd6a2ca-40c2-485c-bc53-db1c652505ed
For more Therapeutic Uses (Complete) data for AMLODIPINE (6 total), please visit the HSDB record page.
In geriatric patients, amlodipine clearance is decreased and AUC is increased by about 40-60%. Therefore, amlodipine dosage should be selected carefully, usually initiating therapy with dosages at the lower end of the recommended range. The greater frequency of decreased hepatic, renal, and/or cardiac function and of concomitant disease and drug therapy observed in the elderly also should be considered.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2008
In patients with hepatic impairment, amlodipine clearance is decreased and AUC is increased by about 40-60%. A reduced initial dosage of the drug is recommended, and subsequent dosage should be titrated slowly.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2008
When amlodipine is used in fixed combination with other drugs (e.g., other antihypertensive agents, atorvastatin), cautions, precautions, contraindications, and interactions associated with the concomitant agent(s) should be considered in addition to those associated with amlodipine. Cautionary information applicable to specific populations (e.g., pregnant or nursing women, individuals with hepatic or renal impairment, geriatric patients) also should be considered for each drug in the fixed combination.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2008
Although some calcium-channel blockers have been shown to worsen the clinical status of patients with heart failure, no evidence of worsening heart failure (based on exercise tolerance, New York Heart Association (NYHA) class, symptoms, or left ventricular ejection fraction) and no adverse effects on overall survival and cardiac morbidity were observed in controlled studies of amlodipine in patients with heart failure. Cardiac morbidity and overall mortality rates in these studies were similar in patients receiving amlodipine and those receiving placebo. In patients with moderate to severe heart failure, amlodipine clearance is decreased and area under the concentration-time curve (AUC) is increased by about 40-60%.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 2008
For more Drug Warnings (Complete) data for AMLODIPINE (15 total), please visit the HSDB record page.
Amlodipine may be used alone or in combination with other antihypertensive and antianginal agents for the treatment of the following conditions: Hypertension Coronary artery disease Chronic stable angina Vasospastic angina (Prinzmetals or Variant angina) Angiographically documented coronary artery disease in patients without heart failure or an ejection fraction < 40%
FDA Label
Treatment of systemic arterial hypertension in cats.
**General pharmacodynamic effects** Amlodipine has a strong affinity for cell membranes, modulating calcium influx by inhibiting selected membrane calcium channels. This drug's unique binding properties allow for its long-acting action and less frequent dosing regimen,. **Hemodynamic effects** After the administration of therapeutic doses of amlodipine to patients diagnosed with hypertension, amlodipine causes vasodilation, which results in a reduction of supine and standing blood pressure. During these blood pressure reductions, there are no clinically significant changes in heart rate or plasma catecholamine levels with long-term use. Acute intravenous administration of amlodipine reduces arterial blood pressure and increases heart rate in patients with chronic stable angina, however, chronic oral administration of amlodipine in clinical studies did not cause clinically significant alterations in heart rate or blood pressures in patients diagnosed with angina and normal blood pressure. With long-term, once daily oral administration, antihypertensive effectiveness is maintained for at least 24 hours. **Electrophysiologic effects** Amlodipine does not change sinoatrial (SA) nodal function or atrioventricular (AV) conduction in animals or humans. In patients who were diagnosed with chronic stable angina, the intravenous administration of 10 mg of amlodipine did not cause clinically significant alterations A-H and H-V conduction and sinus node recovery time after cardiac pacing. Patients administered amlodipine with concomitant beta-blockers produced similar results. In clinical trials in which amlodipine was given in combination with beta-blockers to patients diagnosed with hypertension or angina, no adverse effects on electrocardiographic parameters were noted. In clinical studies comprised of angina patients alone, amlodipine did not change electrocardiographic intervals or produce high degrees of AV block. **Effects on angina** Amlodipine relieves the symptoms of chest pain associated with angina. In patients diagnosed with angina, daily administration of a single amlodipine dose increases total exercise time, the time to angina onset, and the time to 1 mm ST-segment depression on ECG studies, decreases anginal attack frequency, and decreases the requirement for nitroglycerin tablets.
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
QC08CA01
C08CA01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C08 - Calcium channel blockers
C08C - Selective calcium channel blockers with mainly vascular effects
C08CA - Dihydropyridine derivatives
C08CA01 - Amlodipine
Absorption
Amlodipine absorbed slowly and almost completely from the gastrointestinal tract. Peak plasma concentrations are achieved 6-12 hours after oral administration. The estimated bioavailability of amlodipine is 64-90%. Steady-state plasma amlodipine levels are achieved after 7-8 days of consecutive daily dosing. Absorption is not affected by food.
Route of Elimination
Elimination from the plasma occurs in a biphasic with a terminal elimination half-life of about 3050 hours. Steady-state plasma levels of amlodipine are reached after 7-8 days of consecutive daily dosing. Amlodipine is 10% excreted as unchanged drug in the urine. Amlodipine can be initiated at normal doses in patients diagnosed with renal failure,.
Volume of Distribution
21 L/kg,.
Clearance
Total body clearance (CL) has been calculated as 7 1.3 ml/min/kg (0.42 0.078 L/ h/kg) in healthy volunteers,. Elderly patients show a reduced clearance of amlodipine with an AUC (area under the curve) increase of about 4060%, and a lower initial dose may be required.
/MILK/ The aims of this study were to evaluate the plasma concentration of amlodipine and its passage into breast milk in lactating women with pregnancy-induced hypertension and to estimate the risk for breastfeeding infants. Thirty-one lactating women receiving oral amlodipine once daily for pregnancy-induced hypertension were enrolled. Pre-dose plasma and milk concentrations of amlodipine were determined at day 6 or later after starting the medication. Relative infant dose (RID) as an infant risk for breastfeeding was calculated by dividing the infant dose via milk by the maternal dose. The mean maternal dose of amlodipine was 6.0 mg. The medians of the plasma and milk concentrations of amlodipine were 15.5 and 11.5 ng/mL, respectively. Interindividual variation was observed in the amlodipine dose and body weight-adjusted milk concentrations (interquartile range [IQR], 96.7-205 ng/mL per mg/kg). The median and IQR of the amlodipine concentration ratio of milk to plasma were 0.85 and 0.74 to 1.08, respectively. The medians of infant birth weight and daily amlodipine dose via milk were 2170 g and 4.2 ug/kg, respectively. The median of the RID of amlodipine was 4.2% (IQR, 3.1%-7.3%). Lactating women with pregnancy-induced hypertension had higher plasma concentrations of amlodipine during the early postpartum period. Oral amlodipine transferred into breast milk at the same level as that of plasma. However, the RID of amlodipine in most patients was less than 10%.
PMID:25447596 Naito T et al; J Hum Lact 31 (2): 301-6 (2015)
After oral administration of therapeutic doses of Norvasc, absorption produces peak plasma concentrations between 6 and 12 hours. Absolute bioavailability has been estimated to be between 64 and 90%. The bioavailability of Norvasc is not altered by the presence of food.
NIH; DailyMed. Current Medication Information for Norvasc (Amlodipine Besylate) Tablet (Updated: April 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=abd6a2ca-40c2-485c-bc53-db1c652505ed
Steady-state plasma levels of amlodipine are reached after 7 to 8 days of consecutive daily dosing. ... Elderly patients and patients with hepatic insufficiency have decreased clearance of amlodipine with a resulting increase in AUC of approximately 40-60%, and a lower initial dose may be required. A similar increase in AUC was observed in patients with moderate to severe heart failure.
NIH; DailyMed. Current Medication Information for Norvasc (Amlodipine Besylate) Tablet (Updated: April 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=abd6a2ca-40c2-485c-bc53-db1c652505ed
Amlodipine is a dihydropyridine calcium antagonist drug with distinctive pharmacokinetic characteristics which appear to be attributable to a high degree of ionization. Following oral administration, bioavailability is 60 to 65% and plasma concentrations rise gradually to peak 6 to 8 hr after administration. Amlodipine is extensively metabolized in the liver (but there is no significant presystemic or first-pass metabolism) and is slowly cleared with a terminal elimination half-life of 40 to 50 hr. Volume of distribution is large (21 L/kg) and there is a high degree of protein binding (98%). There is some evidence that age, severe hepatic impairment and severe renal impairment influence the pharmacokinetic profile leading to higher plasma concentrations and longer half-lives. There is no evidence of pharmacokinetic drug interactions. Amlodipine shows linear dose-related pharmacokinetic characteristics and, at steady-state, there are relatively small fluctuations in plasma concentrations across a dosage interval. Thus, although structurally related to other dihydropyridine derivatives, amlodipine displays significantly different pharmacokinetic characteristics and is suitable for administration in a single daily dose.
Meredith PA et al; Clin Pharmacokinet 22(1): p.22-31 (1992)
... A randomized, 2-way crossover study was conducted in 18 healthy male volunteers to compare the pharmacokinetics and pharmacodynamics of these two forms, i.e. amlodipine nicotinate (test) and amlodipine besylate (reference), after administration of a single dose of 5 mg of each drug and a washout period between doses of 4 weeks. Blood samples for the pharmacokinetic analysis of amlodipine were obtained over the 144-hour period after administration. Systolic and diastolic blood pressures and pulse rates were recorded immediately prior to each blood sampling. All participants completed both treatment periods, and no serious adverse events occurred during the study period. After administering a single dose of each formulation, mean AUC0-infinity and Cmax values were 190.91+/-60.49 ng x hr/mL and 3.87+/-1.04 ng/mL for the test formulation and 203.15+/-52.05 ng x hr/mL and 4.01+/-0.60 ng/mL for the reference formulation, respectively. The 90% confidence intervals of test/reference mean ratios for AUC0- infinity and Cmax fell within the predetermined equivalence range of 80 - 125%. Pharmacodynamic profiles including systolic and diastolic blood pressures and pulse rates exhibited no significant differences between the two formulations. The two amlodipine formulations showed similar pharmacokinetic and pharmacodynamic characteristics and the new amlodipine formulation, amlodipine nicotinate, was found to be equivalent for pharmacokinetics to the currently available amlodipine besylate with respect to the rate and extent of amlodipine absorption.
PMID:17190374 Park JY et al; Int J Clin Pharmacol Ther 44 (12): 641-7 (2006)
Amlodipine is heavily (approximately 90%) converted to inactive metabolites via hepatic breakdown with 10% of the parent compound and 60% of the metabolites found excreted in the urine. _Ex vivo_ studies have shown that about 93% of the circulating drug is bound to plasma proteins in hypertensive patients. Characteristics that add to amlodipine's unique pharmacologic profile include nearly complete absorption, late-peak plasma concentrations, high bioavailability, and slow hepatic breakdown.
Amlodipine is extensively (about 90%) converted to inactive metabolites via hepatic metabolism with 10% of the parent compound and 60% of the metabolites excreted in the urine.
NIH; DailyMed. Current Medication Information for Norvasc (Amlodipine Besylate) Tablet (Updated: April 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=abd6a2ca-40c2-485c-bc53-db1c652505ed
Metabolism of the dihydropyridine calcium antagonist (R,S)-2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-3-ethoxycarbony l-5- methoxycarbonyl- 6 -methyl- 1,4-dihydropyridine (amlodipine) has been studied in animals and man using (14)C-labelled drug. The metabolite patterns are complex; 18 metabolites have been isolated from rat, dog and human urine. Based on chromatographic and mass-spectral evidence, structures have been proposed for the main metabolites and confirmed by synthesis of unambiguous reference compounds. Comparison of all reference compounds and isolated metabolites was made by gas chromatography-mass spectrometry pressure liquid chromatography on-line thermospray-mass spectrometry of underivatised compounds directly in urine. The metabolites are largely pyridine derivatives. The methods used in structure designation are presented, along with the proposed route of metabolism, which indicates that the metabolic pattern for amlodipine in man has features in common with those of both rat and dog.
PMID:2525041 Beresford AP et al; Arzneimittelforschung 39 (2): 201-9 (1989)
... Objectives of this study were to determine the metabolite profile of amlodipine (a racemic mixture and S-isomer) in human liver microsomes (HLM), and to identify the cytochrome P450 (P450) enzyme(s) involved in the M9 formation. Liquid chromatography/mass spectrometry analysis showed that amlodipine was mainly converted to M9 in HLM incubation. M9 underwent further O-demethylation, O-dealkylation, and oxidative deamination to various pyridine derivatives. This observation is consistent with amlodipine metabolism in humans. Incubations of amlodipine with HLM in the presence of selective P450 inhibitors showed that both ketoconazole (an inhibitor of CYP3A4/5) and CYP3cide (an inhibitor of CYP3A4) completely blocked the M9 formation, whereas chemical inhibitors of other P450 enzymes had little effect. Furthermore, metabolism of amlodipine in expressed human P450 enzymes showed that only CYP3A4 had significant activity in amlodipine dehydrogenation. Metabolite profiles and P450 reaction phenotyping data of a racemic mixture and S-isomer of amlodipine were very similar. The results from this study suggest that CYP3A4, rather than CYP3A5, plays a key role in metabolic clearance of amlodipine in humans.
PMID:24301608 Zhu Y et al; Drug Metab Dispos 42 (2): 245-9 (2014)
In the present study, the metabolic profile of amlodipine, a well-known calcium channel blocker, was investigated employing liquid chromatography-mass spectrometric (LC/MS) techniques. Two different types of mass spectrometers - a triple-quadrupole (QqQ) and a quadrupole time-of-flight (Q-TOF) mass spectrometer - were utilized to acquire structural information on amlodipine metabolites. The metabolites were produced by incubation of amlodipine with primary cultures of rat hepatocytes. Incubations from rat hepatocytes were analyzed with LC-MS/MS, and 21 phase I and phase II metabolites were detected. Their product ion spectra were acquired and interpreted, and structures were proposed. Accurate mass measurement using LC-Q-TOF was used to determine the elemental composition of metabolites and thus to confirm the proposed structures of these metabolites. Mainly phase I metabolic changes were observed including dehydrogenation of the dihydropyridine core, as well as reactions of side chains, such as hydrolysis of ester bonds, hydroxylation, N-acetylation, oxidative deamination, and their combinations. The only phase II metabolite detected was the glucuronide of a dehydrogenated, deaminated metabolite of amlodipine. /Investigators/ propose several in vitro metabolic pathways of amlodipine in rat, based on our analysis of the metabolites detected and characterized.
PMID:18055188 Suchanova B et al; Eur J Pharm Sci 33 (1): 91-9 (2008)
The terminal elimination half-life of about 3050 hours. Plasma elimination half-life is 56 hours in patients with impaired hepatic function, titrate slowly when administering this drug to patients with severe hepatic impairment.
Elimination from the plasma is biphasic with a terminal elimination half-life of about 30-50 hours.
NIH; DailyMed. Current Medication Information for Norvasc (Amlodipine Besylate) Tablet (Updated: April 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=abd6a2ca-40c2-485c-bc53-db1c652505ed
... Following oral administration, /amlodipine has/ a terminal elimination half-life of 40 to 50 hr. ....
Meredith PA et al; Clin Pharmacokinet 22(1): p.22-31 (1992)
**Mechanism of action on blood pressure** Amlodipine is considered a peripheral arterial vasodilator that exerts its action directly on vascular smooth muscle to lead to a reduction in peripheral vascular resistance, causing a decrease in blood pressure. Amlodipine is a dihydropyridine calcium antagonist (calcium ion antagonist or slow-channel blocker) that inhibits the influx of calcium ions into both vascular smooth muscle and cardiac muscle. Experimental studies imply that amlodipine binds to both _dihydropyridine_ and _nondihydropyridine_ binding sites, located on cell membranes. The contraction of cardiac muscle and vascular smooth muscle are dependent on the movement of extracellular calcium ions into these cells by specific ion channels. Amlodipine blocks calcium ion influx across cell membranes with selectivity. A stronger effect of amlodipine is exerted on vascular smooth muscle cells than on cardiac muscle cells. Direct actions of amlodipine on vascular smooth muscle result in reduced blood pressure. **Mechanism of action in angina** The exact mechanism by which amlodipine relieves the symptoms of angina have not been fully elucidated to this date, however, the mechanism of action is likely twofold: Amlodipine has a dilating effect on peripheral arterioles, reducing the total peripheral resistance (afterload) against which the cardiac muscle functions. Since the heart rate remains stable during amlodipine administration, the reduced work of the heart reduces both myocardial energy use and oxygen requirements. Dilatation of the main coronary arteries and coronary arterioles, both in healthy and ischemic areas, is another possible mechanism of amlodipine reduction of blood pressure. The dilatation causes an increase in myocardial oxygen delivery in patients experiencing coronary artery spasm (Prinzmetal's or variant angina) and reduces coronary vasoconstriction caused by smoking.
Amlodipine is a dihydropyridine calcium antagonist (calcium ion antagonist or slow-channel blocker) that inhibits the transmembrane influx of calcium ions into vascular smooth muscle and cardiac muscle. Experimental data suggest that amlodipine binds to both dihydropyridine and nondihydropyridine binding sites. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells than on cardiac muscle cells. Negative inotropic effects can be detected in vitro but such effects have not been seen in intact animals at therapeutic doses. Serum calcium concentration is not affected by amlodipine. Within the physiologic pH range, amlodipine is an ionized compound (pKa=8.6), and its kinetic interaction with the calcium channel receptor is characterized by a gradual rate of association and dissociation with the receptor binding site, resulting in a gradual onset of effect.
NIH; DailyMed. Current Medication Information for Norvasc (Amlodipine Besylate) Tablet (Updated: April 2016). Available from, as of October 31, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=abd6a2ca-40c2-485c-bc53-db1c652505ed
Recent studies have suggested that cytokines are capable of modifying cardiovascular function and that drugs used in the treatment of heart failure have various modulating properties on the production of cytokines. More recently, we have found that ouabain induces the production of cytokines. This study was performed to examine the effects of calcium channel blockers on the production of cytokines induced by a cardiac glycoside. Human peripheral blood mononuclear cells (PBMC) were obtained from healthy volunteers. PBMC were cultured in 0.1, 1, 10, and 30 umol/L amlodipine, diltiazem, and nifedipine in presence of 1 umol/L ouabain. After 24 hr of incubation, IL-1alpha, IL-1beta, IL-6, and TNF-alpha were measured in the culture supernatants by enzyme-linked immunosorbent assay. Ouabain induced the production of IL-1alpha, IL-1beta and IL-6, but not of TNF-alpha. Induction of IL-1beta was most prominent. The production of IL-1alpha, and IL-6 was inhibited by amlodipine in a concentration-dependent manner and was significantly decreased at a concentration of 10 umol/L. IL-1beta production was also inhibited by 30 umol/L amlodipine. In contrast, neither diltiazem nor nifedipine inhibited the production of these cytokines. The unique property of amlodipine to inhibit the production of IL-1alpha, IL-1beta and IL-6 may contribute to its beneficial effects in heart failure patients.
Matsumori A et al; Cytokine 12(3): p.294-297 (2000)
Proliferation of vascular smooth muscle cells (VSMC) contributes to the progression of atherosclerotic plaques. Calcium channel blockers have been shown to reduce VSMC proliferation, but the underlying molecular mechanism remains unclear. p21(Waf1/Cip1) is a potent inhibitor of cell cycle progression. Here, /investigators/ demonstrate that amlodipine (10(-6) to 10(-8) M) activates de novo synthesis of p21(Waf1/Cip1) in vitro. /Investigators/ show that amlodipine-dependent activation of p21(Waf1/Cip1) involves the action of the glucocorticoid receptor (GR) and C/EBP-alpha. The underlying pathway apparently involves the action of mitogen-activated protein kinase or protein kinase C, but not of extracellular signal-related kinase or changes of intracellular calcium. Amlodipine-induced p21(Waf1/Cip1) promoter activity and expression were abrogated by C/EBP-alpha antisense oligonucleotide or by the GR antagonist RU486. Amlodipine-dependent inhibition of cell proliferation was partially reversed by RU486 at 10(-8) M (58%+/-29%), antisense oligonucleotides targeting C/EBP-alpha (91%+/-26%), or antisense mRNAs targeting p21(Waf1/Cip1) (96%+/-32%, n=6); scrambled antisense oligonucleotides or those directed against C/EBP-beta were ineffective. The data suggest that the anti-proliferative action of amlodipine is achieved by induction of the p21 (Waf1/Cip1) gene, which may explain beneficial covert effects of this widely used cardiovascular therapeutic drug beyond a more limited role as a vascular relaxant.
PMID:15466360 Ziesche R et al; FASEB J 18 (13): 1516-23 (2004)
Calcium channel blockers (CCBs) are widely used in the therapy of cardiovascular diseases. Recent studies have shown that several CCBs exerted distinct anti-inflammatory effect in myocardial dysfunction models. The purpose of the present study was to evaluate therapeutic effect and possible mechanism of action of amlodipine, one of the widely used CCBs, on rat cardiac dysfunction during sepsis induced by lipopolysaccharide (LPS). Pretreatment of the rats with amlodipine (10 or 30 mg/kg, i.v.) delayed the fall of mean arterial blood pressure caused by LPS. Amlodipine also significantly inhibited the elevation of plasma tumor necrosis factor alpha (TNF-alpha) and decreased levels of inducible nitric oxide synthase (iNOS) in response to LPS challenge. To investigate the mechanism of the action of amlodipine, neonatal rat cardiomyocytes were used as a model. Amlodipine concentration-dependently decreased the release of TNF-alpha and iNOS protein expression, and suppressed the degradation and phosphorylation of inhibitor of kappaB-alpha (IkappaB-alpha) in LPS-activated neonatal rat cardiomyocytes. Further studies revealed that amlodipine markedly activated phosphatidylinositiol 3-kinase (PI3K) and Akt, downstream of the PI3K signal cascade. Application of PI3K inhibitors, wortmannin and LY294002 attenuated the depression of TNF-alpha and iNOS expression by amlodipine in LPS-induced cardiomyocytes. These findings may explain some cardioprotective effects of amlodipine in LPS-mediated sepsis and suggest that the inhibition of TNF-alpha and iNOS expression by amlodipine is, at least in part, dependent on PI3K/Akt signaling pathway.
PMID:19393774 Li XQ et al; Int Immunopharmacol 9 (9): 1032-41 (2009)

85.3
> 200
838.5k
71.6M
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|---|---|---|
| PAKISTAN | 21,310.00 | 53.4 | > 200 |
| BANGLADESH | 19,558.71 | 51.5 | 51 - 200 |
| TURKEY | 52,255.30 | 80.8 | 51 - 200 |
| INDONESIA | 34,149.20 | 56.1 | 51 - 200 |
| JAPAN | 22,954.37 | 222.4 | 51 - 200 |
| NEPAL | 4,582.00 | 38.6 | 51 - 200 |
| KOREA,REPUBLIC OF | 7,916.12 | 275.6 | 51 - 200 |
| BRAZIL | 39,697.57 | 87.6 | 51 - 200 |
| EGYPT | 9,035.00 | 76.8 | 51 - 200 |
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
24
PharmaCompass offers a list of Amlodipine API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Amlodipine manufacturer or Amlodipine supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Amlodipine manufacturer or Amlodipine supplier.
PharmaCompass also assists you with knowing the Amlodipine API Price utilized in the formulation of products. Amlodipine API Price is not always fixed or binding as the Amlodipine Price is obtained through a variety of data sources. The Amlodipine Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Amlodipine manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Amlodipine, including repackagers and relabelers. The FDA regulates Amlodipine manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Amlodipine API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Amlodipine manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Amlodipine supplier is an individual or a company that provides Amlodipine active pharmaceutical ingredient (API) or Amlodipine finished formulations upon request. The Amlodipine suppliers may include Amlodipine API manufacturers, exporters, distributors and traders.
click here to find a list of Amlodipine suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Amlodipine DMF (Drug Master File) is a document detailing the whole manufacturing process of Amlodipine active pharmaceutical ingredient (API) in detail. Different forms of Amlodipine DMFs exist exist since differing nations have different regulations, such as Amlodipine USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Amlodipine DMF submitted to regulatory agencies in the US is known as a USDMF. Amlodipine USDMF includes data on Amlodipine's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Amlodipine USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Amlodipine suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Amlodipine Drug Master File in Japan (Amlodipine JDMF) empowers Amlodipine API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Amlodipine JDMF during the approval evaluation for pharmaceutical products. At the time of Amlodipine JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Amlodipine suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Amlodipine Drug Master File in Korea (Amlodipine KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Amlodipine. The MFDS reviews the Amlodipine KDMF as part of the drug registration process and uses the information provided in the Amlodipine KDMF to evaluate the safety and efficacy of the drug.
After submitting a Amlodipine KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Amlodipine API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Amlodipine suppliers with KDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Amlodipine as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Amlodipine API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Amlodipine as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Amlodipine and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Amlodipine NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Amlodipine suppliers with NDC on PharmaCompass.
Amlodipine Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Amlodipine GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Amlodipine GMP manufacturer or Amlodipine GMP API supplier for your needs.
A Amlodipine CoA (Certificate of Analysis) is a formal document that attests to Amlodipine's compliance with Amlodipine specifications and serves as a tool for batch-level quality control.
Amlodipine CoA mostly includes findings from lab analyses of a specific batch. For each Amlodipine CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Amlodipine may be tested according to a variety of international standards, such as European Pharmacopoeia (Amlodipine EP), Amlodipine JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Amlodipine USP).
