
Synopsis
Synopsis
0
KDMF
0
VMF
0
Australia

0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
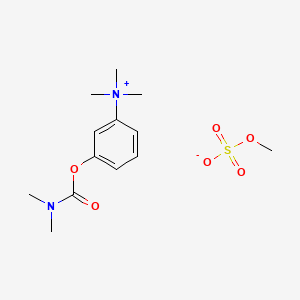

1. Bromide, Neostigmine
2. Methylsulfate, Neostigmine
3. Neostigmine
4. Neostigmine Bromide
5. Polstigmine
6. Proserine
7. Prostigmin
8. Prostigmine
9. Prozerin
10. Synstigmin
11. Syntostigmine
1. Neostigmine Methyl Sulfate
2. 51-60-5
3. Syntostigmin
4. Normastigmin
5. Hodostin
6. Neostigmine Methylsulphate
7. Neostigmine Metilsulfate
8. Neostigmine (methyl Sulfate)
9. Neostigmeth
10. Polstigmine
11. Synstigmine
12. 3-[[(dimethylamino)carbonyl]oxy]-n,n,n-trimethylbenzenaminium Methyl Sulfate
13. Neostigmine Methosulfate
14. Neostigmine Monomethyl Sulfate
15. Eustigmin Methyl Sulfate
16. Proserine Methyl Sulfate
17. Stigmanol Methyl Sulfate
18. Stigmosan Methyl Sulfate
19. Neoeserine Methyl Sulfate
20. Syntostigmin (injection)
21. Leostigmine Methyl Sulfate
22. Kirkstigmine Methyl Sulfate
23. Philostigmin Methyl Sulfate
24. Syntostigmin Methyl Sulfate
25. Vagostigmine Methyl Sulfate
26. Prostigmin
27. Syntostigmine Methyl Sulfate
28. Synthostigmine Methyl Sulfate
29. Intrastigmina
30. Ar-32
31. Sb-23
32. 3-((dimethylcarbamoyl)oxy)-n,n,n-trimethylbenzenaminium Methyl Sulfate
33. Tl-1394
34. (m-hydroxyphenyl)trimethylammonium Methyl Sulfate Dimethylcarbamate
35. Nsc-93753
36. 51-60-5 (methylsulfate)
37. Mls000028383
38. Chebi:7516
39. Chembl211471
40. [3-(dimethylcarbamoxy)phenyl]trimethylammonium Methyl Sulfate
41. [3-(dimethylcarbamoyloxy)phenyl]-trimethylazanium;methyl Sulfate
42. 98imh7m386
43. (3-hydroxyphenyl)trimethylammonium Methyl Sulfate Dimethylcarbamic Ester
44. Juvastigmin
45. Benzenaminium, 3-(((dimethylamino)carbonyl)oxy)-n,n,n-trimethyl-, Methyl Sulfate
46. (3-(dimethylcarbamoxy)phenyl)trimethylammonium Methyl Sulfate
47. Smr000058592
48. Prostigmin (tn)
49. (3-(dimethylcarbamoyl)oxyphenyl)trimethylammonium Methyl Sulfate
50. Syntostigmin (van)
51. Eustigmin Methylsulfate
52. Proserinum
53. Bloxiverz
54. 3-(dimethylcarbamoyloxy)-n,n,n-trimethylbenzenaminium Methyl Sulfate
55. Neostigmine Monomethylsulfate
56. Neostigminmetilsulfat
57. Ccris 3274
58. Gd 65
59. Einecs 200-109-5
60. Nsc 93753
61. Kirkstigmine
62. Proserin
63. [3-(dimethylcarbamoyl)oxyphenyl]trimethylammonium Methyl Sulfate
64. Unii-98imh7m386
65. Bloxiverz (tn)
66. Neostigmine Methylsulfate [usp:jan]
67. Mfcd00011796
68. Neostigmini Metilsulfas
69. Neostigminemethylsulfate
70. 3-(dimethylcarbamoxy)phenyl Trimethylammonium Methyl Sulfate
71. Opera_id_536
72. Prostigmin Methyl Sulfate
73. Ammonium, (3-(dimethylcarbamoyloxy)phenyl)trimethyl-, Methylsulfate
74. Dimethylcarbamic Ester Of 3-oxyphenyltrimethylammonium Methylsulfate
75. Prostigmine Methyl Sulfate
76. Ammonium, (m-hydroxyphenyl)trimethyl-, Methyl Sulfate, Dimethylcarbamate
77. Carbamic Acid, N,n-dimethyl-, 3-dimethylaminophenyl Ester, Methosulfate
78. Neostigmine Methyl Sulphate
79. Carbamic Acid, N,n-dimethyl-, 3-(trimethylammonio)phenyl Ester, Methylsulfate
80. Carbamic Acid, Dimethyl-, Ester With (m-hydroxyphenyl)trimethylammonium Methyl Sulfate
81. Schembl41903
82. Mls001148660
83. Mls001333720
84. Hms500j20
85. Dtxsid40199003
86. Hms2234e04
87. Hms3371e13
88. Act05636
89. Bcp11922
90. Hy-b1206
91. Nsc93753
92. 3-[(dimethylcarbamoyl)oxy]-n,n,n-trimethylanilinium Methyl Sulfate
93. Akos015856682
94. Neostigmine Methylsulfate (jp17/usp)
95. Ac-6847
96. Ccg-213722
97. Cs-4698
98. Neostigmine Methyl Sulfate [mi]
99. Neostigmine Methylsulfate [jan]
100. Neostigmine Metilsulfate [mart.]
101. Neostigmine Methylsulfate [vandf]
102. Neostigmine Metilsulfate [who-dd]
103. Neostigmine Metilsulfate [who-ip]
104. As-15291
105. Neostigmine Methylsulfate [usp-rs]
106. Ammonium, Methyl Sulfate, Dimethylcarbamate
107. Db-051998
108. Benzenaminium,n,n-trimethyl-, Methyl Sulfate
109. Ft-0603223
110. N0447
111. Neostigmine Methylsulfate [green Book]
112. Neostigmini Metilsulfas [who-ip Latin]
113. C08200
114. C75419
115. D00998
116. Neostigmine Methylsulfate [orange Book]
117. Neostigmine Methylsulfate [usp Impurity]
118. Neostigmine Metilsulfate [ep Monograph]
119. Wln: 1n1&vor Ck1&1&1 &q &osw1
120. Neostigmine Methylsulfate [usp Monograph]
121. A828681
122. Q27107519
123. (3-(dimethylcarbamoyloxy)phenyl)trimethyl-ammoniumethylsulfate
124. (3-dimethylcarbamoyloxyphenyl)trimethylammonium Methyl Sulfate
125. (3-hydroxyphenyl)trimethylammonium Methyl Sulfate Dimethylcarbamate
126. [3-(dimethylcarbamoyloxy)phenyl]-trimethyl-azanium; Methyl Sulfate
127. 3-[[(dimethylamino)carbonyl]oxy]-n,n,n-trimethylbenzaminium Bromide
128. Dimethylcarbamic Ester Of 3-oxyphenyltrimethylammonium Methylsulfato
129. (m-hydroxyphenyl)trimethylammonium Methyl Sulphate Dimethylcarbamate
130. [3-[dimethylamino(oxo)methoxy]phenyl]-trimethylammonium; Methyl Sulfate
131. Carbamic Acid, Ester With (m-hydroxyphenyl)trimethylammonium Methyl Sulfate
132. Carbamic Acid,n-dimethyl-, 3-(trimethylammonio)phenyl Ester, Methyl Sulfate
133. Neostigmine Methyl Sulfate, European Pharmacopoeia (ep) Reference Standard
134. Benzenaminium, 3-(((dimethylamino)carbonyl)oxy)-n,n,n-trimethyl-, Methyl Sulphate
135. Neostigmine Methyl Sulfate, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 334.39 g/mol |
|---|---|
| Molecular Formula | C13H22N2O6S |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 334.11985760 g/mol |
| Monoisotopic Mass | 334.11985760 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 337 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Bloxiverz |
| PubMed Health | Neostigmine |
| Drug Classes | Central Nervous System Agent, Immunological Agent, Nondepolarizing Muscle Relaxant Antagonist |
| Drug Label | Neostigmine methylsulfate, a cholinesterase inhibitor, is (m-hydroxyphenyl) trimethylammonium methylsulfate dimethylcarbamate. The structural formula is:Neostigmine methylsulfate is a white crystalline powder and is very soluble in water and soluble... |
| Active Ingredient | Neostigmine methylsulfate |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 10mg/10ml (1mg/ml); 5mg/10ml (0.5mg/ml) |
| Market Status | Prescription |
| Company | Eclat Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Bloxiverz |
| PubMed Health | Neostigmine |
| Drug Classes | Central Nervous System Agent, Immunological Agent, Nondepolarizing Muscle Relaxant Antagonist |
| Drug Label | Neostigmine methylsulfate, a cholinesterase inhibitor, is (m-hydroxyphenyl) trimethylammonium methylsulfate dimethylcarbamate. The structural formula is:Neostigmine methylsulfate is a white crystalline powder and is very soluble in water and soluble... |
| Active Ingredient | Neostigmine methylsulfate |
| Dosage Form | Solution |
| Route | Intravenous |
| Strength | 10mg/10ml (1mg/ml); 5mg/10ml (0.5mg/ml) |
| Market Status | Prescription |
| Company | Eclat Pharms |
Cholinesterase Inhibitors
Drugs that inhibit cholinesterases. The neurotransmitter ACETYLCHOLINE is rapidly hydrolyzed, and thereby inactivated, by cholinesterases. When cholinesterases are inhibited, the action of endogenously released acetylcholine at cholinergic synapses is potentiated. Cholinesterase inhibitors are widely used clinically for their potentiation of cholinergic inputs to the gastrointestinal tract and urinary bladder, the eye, and skeletal muscles; they are also used for their effects on the heart and the central nervous system. (See all compounds classified as Cholinesterase Inhibitors.)
Parasympathomimetics
Drugs that mimic the effects of parasympathetic nervous system activity. Included here are drugs that directly stimulate muscarinic receptors and drugs that potentiate cholinergic activity, usually by slowing the breakdown of acetylcholine (CHOLINESTERASE INHIBITORS). Drugs that stimulate both sympathetic and parasympathetic postganglionic neurons (GANGLIONIC STIMULANTS) are not included here. (See all compounds classified as Parasympathomimetics.)

API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?