
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
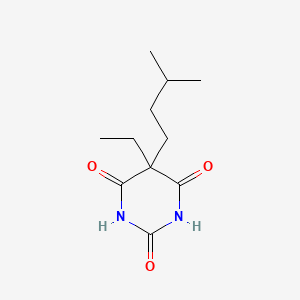

1. Amobarbital Sodium
2. Amobarbital, Sodium
3. Amsal
4. Amylbarb Sodium
5. Amylobarbitone
6. Amylobeta
7. Amytal
8. Amytal Sodium
9. Barbamyl
10. Eunoctal
11. Isoamitil Sedante
12. Isonal
13. Neur-amyl
14. Novamobarb
15. Pentymal
16. Placidel
17. Sodium Amobarbital
18. Sodium Amytal
19. Sodium, Amobarbital
20. Transital
1. Amylobarbitone
2. Barbamyl
3. Isomytal
4. Amytal
5. Amylobarbital
6. 57-43-2
7. Barbamil
8. Pentymal
9. Amobarbitone
10. Amylbarbitone
11. Eunoctal
12. Talamo
13. Barbamyl Acid
14. Binoctal
15. Dorlotyn
16. Dormytal
17. Mylodorm
18. Pentymalum
19. Schiwanox
20. Sednotic
21. Stadadorm
22. Amasust
23. Amital
24. Amospan
25. Amybal
26. Robarb
27. Somnal
28. Sumital
29. Isomyl
30. Amal
31. Isoamylethylbarbituric Acid
32. 5-ethyl-5-isopentylbarbituric Acid
33. Ethylisopentylbarbituric Acid
34. 5-ethyl-5-isoamylbarbituric Acid
35. 5-ethyl-5-isoamylmalonyl Urea
36. 5-ethyl-5-(3-methylbutyl)barbituric Acid
37. 5-isoamyl-5-ethylbarbituric Acid
38. Barbituric Acid, 5-ethyl-5-isopentyl-
39. Nsc 10815
40. 5-ethyl-5-(3-methylbutyl)-2,4,6(1h,3h,5h)-pyrimidinetrione
41. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-ethyl-5-(3-methylbutyl)-
42. Amobarbital Cii
43. 5-ethyl-5-(3-methylbutyl)-1,3-diazinane-2,4,6-trione
44. Component Of Dexamyl
45. Component Of Q-caps
46. Component Of 15-90
47. Nsc-10815
48. Component Of Amo-dextrosule
49. Gwh6ij239e
50. Chebi:2673
51. 5-ethyl-5-(3-methylbutyl)pyrimidine-2,4,6(1h,3h,5h)-trione
52. Ncgc00247711-01
53. Amobarbitale
54. Amobarbitalum
55. Amobarbitale [dcit]
56. Amobarbitalum [inn-latin]
57. 5-ethyl-5-isopentylbarbitursaeure
58. Dea No. 2125
59. Wln: T6vmvmv Fhj F2 F2y1&1
60. Isomytal (tn)
61. Ccris 5454
62. Hsdb 3286
63. Einecs 200-330-7
64. Unii-gwh6ij239e
65. 2,6(1h,3h,5h)-pyrimidinetrione, 5-ethyl-5-(3-methylbutyl)-
66. 5-ethyl-5-(3-methylbutyl)-2,6(1h,3h,5h)-pyrimidinetrione
67. Amobarbital [usp:inn:ban:jan]
68. Dexamyl (salt/mix)
69. Dsstox_cid_81
70. Amobarbital [mi]
71. Amobarbital [inn]
72. Amobarbital [jan]
73. Amobarbital [hsdb]
74. Amobarbital (jp17/inn)
75. Amobarbital [vandf]
76. Amobarbital [mart.]
77. Dsstox_rid_75353
78. Bidd:pxr0091
79. Dsstox_gsid_20081
80. Oprea1_587446
81. Schembl43780
82. Amobarbital [who-dd]
83. Cbdive_006514
84. Divk1c_000994
85. Chembl267894
86. Dtxsid9020081
87. Schembl15364946
88. Component Of Dexamyl (salt/mix)
89. Kbio1_000994
90. Virovyvqcglcii-uhfffaoysa-
91. Amobarbital [ep Impurity]
92. Amobarbital Cii [usp-rs]
93. Component Of Q-caps (salt/mix)
94. Ninds_000994
95. Component Of 15-90 (salt/mix)
96. Amobarbital 0.1 Mg/ml In Methanol
97. Amobarbital 1.0 Mg/ml In Methanol
98. Nsc10815
99. Nsc32406
100. Zinc4811698
101. Tox21_112866
102. Nsc-32406
103. Nsc120800
104. Barbituric Acid, 5-ethyl-5-isoamyl-
105. Db01351
106. Nsc-120800
107. Cas-57-43-2
108. Component Of Amo-dextrosule (salt/mix)
109. Idi1_000994
110. C07536
111. D00555
112. 057a558
113. Q415850
114. 5-ethyl-5-isopentyl-2,4,6(1h,3h,5h)-pyrimidinetrione #
115. Amobarbital Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 226.27 g/mol |
|---|---|
| Molecular Formula | C11H18N2O3 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 226.13174244 g/mol |
| Monoisotopic Mass | 226.13174244 g/mol |
| Topological Polar Surface Area | 75.3 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 303 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
GABA Modulators; Sedatives, Barbiturate
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Amobarbital (no longer commercially available in the US) and amobarbital sodium are used principally as hypnotics in the short-term treatment of insomnia for periods up two two weeks in duration ... The drugs are also used for routine sedation and to relieve anxiety and provide sedation preoperatively.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2580
Amobarbital sodium may be used ... to control acute episodes of agitated behavior in psychoses such as catatonic, negativistic, or manic reactions, but has little value in longterm management of psychoses. Parenteral amobarbital sodium may also be useful in narcoanalysis, narcotherapy, and as a diagnostic aid in schizophrenia.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2580
/Experimental Therapy:/ ... Presurgical screening for temporal lobe epilepsy (TLE) includes the intracarotid amobarbital procedure (IAP), consisting of two consecutive injections of amobarbital, ipsilateral and contralateral to the epileptic focus. We studied whether a bilateral IAP has added value to a unilateral, ipsilateral IAP. METHODS: This population-based study included 183 consecutive patients referred for screening for TLE surgery who underwent bilateral IAP. Using multivariable modeling, we assessed the added value of bilateral IAP on the decision for surgery, resection size, amygdalohippocampectomy, post-operative seizure freedom, memory performance, and IQ change. RESULTS: Given the results from the unilateral IAP, the bilateral IAP had added prognostic value for postoperative change in verbal memory (P < 0.01) and verbal IQ (P < 0.01), especially if patients had a left-sided focus. In contrast, information provided by the contralateral IAP was not associated with decision-making or surgical strategy. CONCLUSIONS: A bilateral IAP has added value in predicting post-operative verbal memory and IQ. A bilateral IAP is currently not used to guide surgical strategy, but may be used for this purpose when verbal capacity is of particular concern in patients with a left-sided focus. In other cases, IAP is best performed unilaterally.
PMID:18684215 Uijl SG et al; Acta Neurol Scand 119 (3): 199-206 (2009).
Medication (Vet): ... used primarily for its hypnotic or sedative action. /Amobarbital sodium/
Booth, N.H., L.E. McDonald (eds.). Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press, 1982., p. 257
Amobarbital (no longer commercially available in the US) and amobarbital sodium share the toxic potentials of the barbiturates, and the usual precautions of barbiturate administration should be observed.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2580
Dependence of the barbiturate-alcohol type is liable to occur in susceptible patients given any of the sedatives & hypnotics ... . It is characterized by a strong need to continue taking the drug, a tendency to increase the dose, a psychic dependence on the effects of the drug, and a physical dependence on the effects of the drug for the maintenance of homoeostasis, with a characteristic abstinence syndrome on withdrawal. /Hypnotics & sedatives/
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 792
Vet: avoid use of simultaneously administered chloramphenicol which has caused a dramatic increase in anesthesia duration in mice.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 17
IV administered amobarbital sodium may cause respiratory depression, apnea, or hypotension, particularly if the drug is administered too rapidly. The drug must be administered slowly at a rate not greater than 100 mg/minute, and personnel and equipment should be readily available for administration of artificial respiration. Safety & efficacy of amobarbital & amobarbital sodium in children younger than 6 yr of age have not been established.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2580
For more Drug Warnings (Complete) data for Amobarbital (29 total), please visit the HSDB record page.
The toxic dose of barbiturates varies considerably but, in general, a severe reaction is likely to occur when the amount ingested is more than 10 times the usual oral hypnotic dose. Potentially lethal blood concentrations are those in excess of 80 ug/mL for phenobarbital, 50 ug/mL for amobarbital or butabarbital, and approximately 30 ug/mL for secobarbital or pentobarbital; however, some patients have survived much higher blood concentrations. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2578
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CA - Barbiturates, plain
N05CA02 - Amobarbital
Following oral or rectal admin, the onset of action varies from 10-30 min for ... amobarbital ... Following iv admin of the sodium salts of ... amobarbital, the onset of action ranges from almost immediately for pentobarbital ... .
American Hospital Formulary Service - Drug Information 89. Bethesda, MD: American Society of Hospital Pharmacists, 1989 (Plus Supplements)., p. 1185
About 40 to 60% of amylobarbitone is bound to plasma proteins.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 793
Following admin of radioactively labelled amylobarbitone to 2 healthy subjects 79 to 92% was recovered in urine in 6 days and only 4 to 5% in the feces. Unchanged drug was practically absent from both urine and feces.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 793
Fluid & tissue specimens collected from 30 subjects at autopsy were assayed for amylobarbitone (amobarbital), butobarbitone (butethal), pentobarbitone (pentobarbital), quinalbarbitone (secobarbital) and the corresponding hydroxylated metabolites by GLC. Where one barbiturate was ingested, an inverse relationship between lipid solubility of the drug and the distribution in fluids and tissues was observed. In most cases the liver, and in the remainder the spleen, contained the highest concn of barbiturate. Bile concn were often in excess of those in the corresponding liver. The metabolites of the 4 sedative barbiturates were usually present in lower amounts than the parent drugs in the fluids and tissues of most subjects, but urine often contained much higher concn of metabolites, sometimes exceeding that of the parent drug in the liver. Admin of 2 or more barbiturates together did not appear to affect the distribution and metabolism of the individual drugs.
PMID:39132 Robinson AE, McDowall RD; J Pharm Pharmacol 31: 357-65 (1979)
For more Absorption, Distribution and Excretion (Complete) data for Amobarbital (17 total), please visit the HSDB record page.
Amobarbital is metabolized by the liver via penultimate oxidation of the 3-methylbutyl substituent to form a tertiary alcohol, hydroxyamobarbital, which is an inactive metabolite. About 40-50% of an oral hypnotic dose of amobarbital is excreted in urine as hydroxyamobarbital and its glucuronide conjugates. Less than 1% of an oral hypnotic dose of the drug is excreted in urine unchanged. Conjugates of hydroxyamobarbital excreted in feces or urine and/or further oxidation products not yet identified may account for the remainder of the dose.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2580
Following admin of radioactively labelled amylobarbitone to two healthy subjects ... less than 50% of the dose was identified as 3'-hydroxyamylobarbitone. A second main metabolite was identified as N-hydroxyamylobarbitone and was found to account for up to 30% of the dose.
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 793
Relative proportion of amobarbital metabolites in urine is highly variable and observations of plasma half-life give no indication of this variability. A valid estimate of a given person's metabolite pattern can be obtained by studying single urine specimen in postdistributive phase. Two metabolites were measured in urine specimen in the postdistributive phase. The 2 metabolites were 3'-hydroxyamobarbital as product of side chain hydroxylation and N-beta-d-glycopyranosyl amobarbital, a glucose conjugate.
PMID:699482 Kalow W et al; Clin Pharmacol Ther 24 (5): 576-82 (1978)
N-Hydroxyamylobarbitone was synthesized and its occurrence as a urinary metabolite was studied in 13 subjects taking 200 mg sodium amobarbital. It was concluded that the excretion of free N-hydroxyamylobarbitone is not significant in the metabolism of amobarbital sodium. /Amobarbital sodium/
Gilbert JN et al; J Pharm Pharmacol 30 (9) 595 (1978)
For more Metabolism/Metabolites (Complete) data for Amobarbital (8 total), please visit the HSDB record page.
Following bolus iv administration, plasma concentrations of amobarbital decline in a biphasic manner with a half-life of about 40 minutes for the first phase and 20-25 hours for the second phase, although the second phase half-life has ranged from 14-42 hours in individual patients.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2580
The half-life of amobarbital was investigated in 36 unrelated subjects; half-life (23.8 hr) appeared to be normally distributed.
PMID:975717 Inaba T et al; Clin Pharmacol Ther 20 (4): 439-44 (1976)
Following im injection of amylobarbitone sodium 200 mg to mildly hypertensive women in labor 0.7 to 3.5 hr before delivery, the plasma-half-life of amylobarbitone was 2.5 times as long in the neonates as in the mothers. /Amobarbital sodium/
Reynolds, J.E.F., Prasad, A.B. (eds.) Martindale-The Extra Pharmacopoeia. 28th ed. London: The Pharmaceutical Press, 1982., p. 793
Amobarbital (like all barbiturates) works by binding to the GABAA receptor at either the alpha or the beta sub unit. These are binding sites that are distinct from GABA itself and also distinct from the benzodiazepine binding site. Like benzodiazepines, barbiturates potentiate the effect of GABA at this receptor. This GABAA receptor binding decreases input resistance, depresses burst and tonic firing, especially in ventrobasal and intralaminar neurons, while at the same time increasing burst duration and mean conductance at individual chloride channels; this increases both the amplitude and decay time of inhibitory postsynaptic currents. In addition to this GABA-ergic effect, barbiturates also block the AMPA receptor, a subtype of glutamate receptor. Glutamate is the principal excitatory neurotransmitter in the mammalian CNS. Amobarbital also appears to bind neuronal nicotinic acetylcholine receptors.
The exact mechanism(s) by which barbiturates exert their effect on the CNS, has not been fully elucidated. However, it is believed that such effects are related, at least partially, to the drugs' ability to enhance the activity of gamma-aminobutyric acid (GABA), the principal inhibitory neurotransmitter in the CNS, by altering inhibitory synaptic transmissions that are mediated by GABAA receptors. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Although the drugs act throughout the CNS, a site of particular sensitivity is the polysynaptic midbrain reticular formation which is concerned with the arousal mechanism. Barbiturates induce an imbalance in central inhibitory and facilitatory mechanisms influencing the cerebral cortex and the reticular formation. The significance of the effect of barbiturates on neurotransmitters is unclear. It appears that the drugs decrease the excitability of both presynaptic and postsynaptic membranes. It has not been determined which of the various actions of barbiturates at cellular and synaptic levels are responsible for their sedative and hypnotic effects. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Relatively low doses of the barbiturates depress the sensory cortex, decrease motor activity, and produce sedation and drowsiness. In some patients, however, drowsiness may be preceded by a period of transient elation, confusion, euphoria, or excitement, especially after subhypnotic doses of aprobarbital, pentobarbital, or secobarbital. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Larger doses distort judgment, cloud perception, suppress motor activity, and produce drowsiness and sleep. Still larger doses induce anesthesia. Barbiturate-induced sleep differs from physiologic sleep. Barbiturates reduce the rapid eye movement (REM) or dreaming stage of sleep. Stages III and IV sleep are also decreased. Although tolerance develops to the REM-suppressant effects during chronic administration, REM rebound occurs when the drugs are withdrawn, and the patient may experience markedly increased dreaming, nightmares, and/or insomnia. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
For more Mechanism of Action (Complete) data for Amobarbital (18 total), please visit the HSDB record page.
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
59
PharmaCompass offers a list of Amobarbital API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Amobarbital manufacturer or Amobarbital supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Amobarbital manufacturer or Amobarbital supplier.
PharmaCompass also assists you with knowing the Amobarbital API Price utilized in the formulation of products. Amobarbital API Price is not always fixed or binding as the Amobarbital Price is obtained through a variety of data sources. The Amobarbital Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Amobarbital manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Amobarbital, including repackagers and relabelers. The FDA regulates Amobarbital manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Amobarbital API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Amobarbital manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Amobarbital supplier is an individual or a company that provides Amobarbital active pharmaceutical ingredient (API) or Amobarbital finished formulations upon request. The Amobarbital suppliers may include Amobarbital API manufacturers, exporters, distributors and traders.
click here to find a list of Amobarbital suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Amobarbital Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Amobarbital GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Amobarbital GMP manufacturer or Amobarbital GMP API supplier for your needs.
A Amobarbital CoA (Certificate of Analysis) is a formal document that attests to Amobarbital's compliance with Amobarbital specifications and serves as a tool for batch-level quality control.
Amobarbital CoA mostly includes findings from lab analyses of a specific batch. For each Amobarbital CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Amobarbital may be tested according to a variety of international standards, such as European Pharmacopoeia (Amobarbital EP), Amobarbital JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Amobarbital USP).
