
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
US Medicaid
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
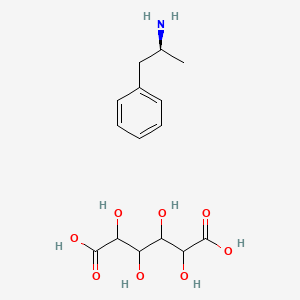

1. Dextroamphetamine Saccharate
| Molecular Weight | 345.34 g/mol |
|---|---|
| Molecular Formula | C15H23NO8 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | 345.14236669 g/mol |
| Monoisotopic Mass | 345.14236669 g/mol |
| Topological Polar Surface Area | 182 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 287 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 4 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
GDUFA
DMF Review : Complete
Rev. Date : 2013-04-10
Pay. Date : 2013-04-03
DMF Number : 24071
Submission : 2010-08-10
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15090
Submission : 2000-10-13
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2013-01-11
Pay. Date : 2012-12-12
DMF Number : 15305
Submission : 2001-02-26
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2014-01-03
Pay. Date : 2013-11-21
DMF Number : 16022
Submission : 2002-06-21
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15608
Submission : 2001-09-01
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2013-06-08
Pay. Date : 2013-05-31
DMF Number : 15428
Submission : 2001-05-15
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2016-08-31
Pay. Date : 2015-08-27
DMF Number : 29603
Submission : 2016-02-25
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2023-11-06
Pay. Date : 2023-08-03
DMF Number : 38543
Submission : 2023-08-01
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AB
AMPHETAMINE ASPARTATE; AMPHETAMINE SULFATE; DEXTROAMPHETAMINE SACCHARATE; DEXTROAMPHETAMINE SULFATE
Brand Name : DEXTROAMP SACCHARATE, AMP ASPARTATE, DEXTROAMP SULFATE AND AMP SULFATE
Dosage Form : TABLET;ORAL
Dosage Strength : 1.25MG;1.25MG;1.25MG;1.25MG
Approval Date : 2021-12-28
Application Number : 215771
RX/OTC/DISCN : RX
RLD : No
TE Code : AB
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AB
AMPHETAMINE ASPARTATE; AMPHETAMINE SULFATE; DEXTROAMPHETAMINE SACCHARATE; DEXTROAMPHETAMINE SULFATE
Brand Name : DEXTROAMP SACCHARATE, AMP ASPARTATE, DEXTROAMP SULFATE AND AMP SULFATE
Dosage Form : TABLET;ORAL
Dosage Strength : 1.875MG;1.875MG;1.875MG;1.875MG
Approval Date : 2021-12-28
Application Number : 215771
RX/OTC/DISCN : RX
RLD : No
TE Code : AB
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AB
AMPHETAMINE ASPARTATE; AMPHETAMINE SULFATE; DEXTROAMPHETAMINE SACCHARATE; DEXTROAMPHETAMINE SULFATE
Brand Name : DEXTROAMP SACCHARATE, AMP ASPARTATE, DEXTROAMP SULFATE AND AMP SULFATE
Dosage Form : TABLET;ORAL
Dosage Strength : 2.5MG;2.5MG;2.5MG;2.5MG
Approval Date : 2021-12-28
Application Number : 215771
RX/OTC/DISCN : RX
RLD : No
TE Code : AB
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AB
AMPHETAMINE ASPARTATE; AMPHETAMINE SULFATE; DEXTROAMPHETAMINE SACCHARATE; DEXTROAMPHETAMINE SULFATE
Brand Name : DEXTROAMP SACCHARATE, AMP ASPARTATE, DEXTROAMP SULFATE AND AMP SULFATE
Dosage Form : TABLET;ORAL
Dosage Strength : 3.75MG;3.75MG;3.75MG;3.75MG
Approval Date : 2021-12-28
Application Number : 215771
RX/OTC/DISCN : RX
RLD : No
TE Code : AB
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AB
AMPHETAMINE ASPARTATE; AMPHETAMINE SULFATE; DEXTROAMPHETAMINE SACCHARATE; DEXTROAMPHETAMINE SULFATE
Brand Name : DEXTROAMP SACCHARATE, AMP ASPARTATE, DEXTROAMP SULFATE AND AMP SULFATE
Dosage Form : TABLET;ORAL
Dosage Strength : 5MG;5MG;5MG;5MG
Approval Date : 2021-12-28
Application Number : 215771
RX/OTC/DISCN : RX
RLD : No
TE Code : AB
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AB
AMPHETAMINE ASPARTATE; AMPHETAMINE SULFATE; DEXTROAMPHETAMINE SACCHARATE; DEXTROAMPHETAMINE SULFATE
Brand Name : DEXTROAMP SACCHARATE, AMP ASPARTATE, DEXTROAMP SULFATE AND AMP SULFATE
Dosage Form : TABLET;ORAL
Dosage Strength : 7.5MG;7.5MG;7.5MG;7.5MG
Approval Date : 2021-12-28
Application Number : 215771
RX/OTC/DISCN : RX
RLD : No
TE Code : AB
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AB1
AMPHETAMINE ASPARTATE; AMPHETAMINE SULFATE; DEXTROAMPHETAMINE SACCHARATE; DEXTROAMPHETAMINE SULFATE
Brand Name : DEXTROAMP SACCHARATE, AMP ASPARTATE, DEXTROAMP SULFATE AND AMP SULFATE
Dosage Form : CAPSULE, EXTENDED RELEASE;ORAL
Dosage Strength : 1.25MG;1.25MG;1.25MG;1.25MG
Approval Date : 2023-01-23
Application Number : 217027
RX/OTC/DISCN : RX
RLD : No
TE Code : AB1
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AB1
AMPHETAMINE ASPARTATE; AMPHETAMINE SULFATE; DEXTROAMPHETAMINE SACCHARATE; DEXTROAMPHETAMINE SULFATE
Brand Name : DEXTROAMP SACCHARATE, AMP ASPARTATE, DEXTROAMP SULFATE AND AMP SULFATE
Dosage Form : CAPSULE, EXTENDED RELEASE;ORAL
Dosage Strength : 2.5MG;2.5MG;2.5MG;2.5MG
Approval Date : 2023-01-23
Application Number : 217027
RX/OTC/DISCN : RX
RLD : No
TE Code : AB1
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AB1
AMPHETAMINE ASPARTATE; AMPHETAMINE SULFATE; DEXTROAMPHETAMINE SACCHARATE; DEXTROAMPHETAMINE SULFATE
Brand Name : DEXTROAMP SACCHARATE, AMP ASPARTATE, DEXTROAMP SULFATE AND AMP SULFATE
Dosage Form : CAPSULE, EXTENDED RELEASE;ORAL
Dosage Strength : 3.75MG;3.75MG;3.75MG;3.75MG
Approval Date : 2023-01-23
Application Number : 217027
RX/OTC/DISCN : RX
RLD : No
TE Code : AB1
 Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
Granules India Limited has high volume world-class facilities for APIs, PFIs, & FDFs, serving customers in over 80 countries.
RLD : No
TE Code : AB1
AMPHETAMINE ASPARTATE; AMPHETAMINE SULFATE; DEXTROAMPHETAMINE SACCHARATE; DEXTROAMPHETAMINE SULFATE
Brand Name : DEXTROAMP SACCHARATE, AMP ASPARTATE, DEXTROAMP SULFATE AND AMP SULFATE
Dosage Form : CAPSULE, EXTENDED RELEASE;ORAL
Dosage Strength : 5MG;5MG;5MG;5MG
Approval Date : 2023-01-23
Application Number : 217027
RX/OTC/DISCN : RX
RLD : No
TE Code : AB1
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Application : Fillers, Diluents & Binders, Taste Masking
Excipient Details : Mannitol is used as a filler, bulking agent and taste masking agent in ODT formulations such as tablets.
Dosage Form : Cream / Lotion / Ointment, Emulsion, Gel, Solution, Tablet
Grade : Topical, Oral
Category : API Stability Enhancers, Emulsifying Agents, Fillers, Diluents & Binders
Dosage Form : Granule / Pellet, Tablet
Grade : Oral
Category : Fillers, Diluents & Binders, Granulation, Solubilizers
Dosage Form : Capsule, Granule / Pellet, Tablet, Injectable / Parenteral
Grade : Parenteral, Oral
Category : Fillers, Diluents & Binders, Thickeners and Stabilizers
Application : Co-Processed Excipients
Excipient Details : Mannitol is a co-processed excipient with superior compactibility, lower disintegration time, and hygroscopicity.
Application : Fillers, Diluents & Binders
Excipient Details : KoVidone® K25 is used as a low viscosity wet binder in solid dosage forms such as capsules and tablets.
Pharmacopoeia Ref : USP/NF, EP, JP, KP, IP, BP
Technical Specs : NA
Ingredient(s) : Polyvinylpyrrolidone
Dosage Form : Capsule, Granule / Pellet, Tablet
Grade : Oral
Category : Coating Systems & Additives, Controlled & Modified Release, Solubilizers
Brand Name : EUDRAGIT® L 100-55
Application : Coating Systems & Additives, Controlled & Modified Release, Solubilizers
Excipient Details : EUDRAGIT® L 100-55 (powder) is used in delayed release coatings to enhance solubility of poorly soluble drugs such as tablets, capsules & granules.
Pharmacopoeia Ref : NA
Technical Specs : NA
Ingredient(s) : Methacrylic Acid - Ethyl Acrylate Copolymer
Dosage Form : Tablet
Grade : Oral
Category : Coating Systems & Additives, Controlled & Modified Release
Dosage Form : Capsule, Granule / Pellet, Tablet
Grade : Oral
Category : Coating Systems & Additives, Coloring Agents
Brand Name : Titanium dioxide PRETIOX AV01FG
Application : Coating Systems & Additives, Coloring Agents
Excipient Details : Titanium dioxide Pretiox AV01FG is used as a coloring and coating agent in oral solid dosage forms such as capsules, tablets, granules, and pellets.
Pharmacopoeia Ref : Fami-QS, Kosher, Halal, OHSAS ...
Technical Specs : Ti 59.95% and O 40.05%
Ingredient(s) : Titanium Dioxide
Dosage Form : Capsule, Granule / Pellet, Tablet
Grade : Oral
Category : Coating Systems & Additives, Coloring Agents
Grade : Oral
Category : Coating Systems & Additives, Film Formers & Plasticizers
Brand Name : EUDRAGIT® L 30 D-55
Application : Coating Systems & Additives, Film Formers & Plasticizers
Excipient Details : EUDRAGIT® L 30 D-55 (aqueous dispersion) is a delayed release polymer used as coating and film former in tablets, capsules, granules etc.
Pharmacopoeia Ref : NA
Technical Specs : NA
Ingredient(s) : Methacrylic Acid - Ethyl Acrylate Copolymer
Grade : Not Available
Category : Coating Systems & Additives, Controlled & Modified Release
Application : Coating Systems & Additives, Controlled & Modified Release
Excipient Details : Film Forming Agent, Wet/Dry Granulation- Binder,Thickening & Suspension Agent, Non-Gelatin Capsule Manufacturing & Enteric Film Coating Systems
Pharmacopoeia Ref : USP, EP, and JP
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Brand Name : Hydroxypropyl Methyl Cellulose
Application : Coating Systems & Additives
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Emulsion, Tablet
Grade : Oral, Ophthalmic
Category : Disintegrants & Superdisintegrants, Emulsifying Agents, Film Formers & Plasticizers, Thickeners and Stabilizers
Brand Name : Hydroxypropyl Methyl Cellulose
Application : Disintegrants & Superdisintegrants, Emulsifying Agents, Film Formers & Plasticizers, Thickeners and Stabilizers
Excipient Details : Hydroxypropyl Methyl Cellulose is used as a film-former, disintegrant, thickener, and emulsifier in tablets, emulsions, and ophthalmic formulations.
Grade : Not Available
Category : Coating Systems & Additives, Film Formers & Plasticizers
Brand Name : ReadiLYCOAT® D CLEAR 110.01
Application : Coating Systems & Additives, Film Formers & Plasticizers
Excipient Details : A natural inert polymer and ready-to-use coating system for fast aqueous film coating saves up to 50% or more time.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Pea Starch, Sorbitol, Stearic Acid
Dosage Form : Tablet
Grade : Oral
Category : Coating Systems & Additives, Controlled & Modified Release
Dosage Form : Cream / Lotion / Ointment, Gel, Injectable / Parenteral, Softgel Capsule, Softgels
Grade : Parenteral, Topical, Oral
Category : Fillers, Diluents & Binders, Film Formers & Plasticizers, Parenteral, Surfactant & Foaming Agents, Thickeners and Stabilizers, Topical
Brand Name : Polyethylene Glycol 400
Application : Fillers, Diluents & Binders, Film Formers & Plasticizers, Parenteral, Surfactant & Foaming Agents, Thickeners and Stabilizers, Topical
Excipient Details : PEG 400 is used as a suspending agent, stabilizer, plasticizer and filler in OSDs, liquids & semi-solids and as a solvent for parenteral formulations.
Dosage Form : Capsule, Granule / Pellet, Tablet
Grade : Oral
Category : Coating Systems & Additives, Controlled & Modified Release, Solubilizers
Brand Name : EUDRAGIT® L 100-55
Application : Coating Systems & Additives, Controlled & Modified Release, Solubilizers
Excipient Details : EUDRAGIT® L 100-55 (powder) is used in delayed release coatings to enhance solubility of poorly soluble drugs such as tablets, capsules & granules.
Pharmacopoeia Ref : NA
Technical Specs : NA
Ingredient(s) : Methacrylic Acid - Ethyl Acrylate Copolymer
Dosage Form : Tablet
Grade : Oral
Category : Coating Systems & Additives, Controlled & Modified Release
Application : Coating Systems & Additives, Controlled & Modified Release
Excipient Details : It is a dry powder derived from a 30% aqueous dispersion (ECOPOL L 30 D 55). The major application is for Enteric/Delayed release coating for drug delivery in the duodenum.
Pharmacopoeia Ref : Monograph - EP, USP, JPE
Technical Specs : Not Available
Ingredient(s) : Methacrylic Acid - Ethyl Acrylate Copolymer
Application : Controlled & Modified Release
Excipient Details : PLLA-PEG used in the synthesis of targeted nanoparticles which are used for differential delivery and controlled release of drugs.
Pharmacopoeia Ref : NA
Technical Specs : Nano-particles, ultrapure, low-monomer & powder grades
Ingredient(s) : Poly L Lactide
Dosage Form : Capsule, Tablet, Topical Film, Transdermal Patch
Grade : Not Available
Category : Controlled & Modified Release, Direct Compression, Granulation
Application : Controlled & Modified Release, Direct Compression, Granulation
Excipient Details : For non-erodible matrices using direct compression, Controlled release matrix. Matrix former in transdermal patches and topical films.
Pharmacopoeia Ref : Ph. Eur., USP-NF, JP-JPE: 80 %...
Technical Specs : Not Available
Ingredient(s) : Lauryl Sulfate
Grade : Not Available
Category : Coating Systems & Additives, Controlled & Modified Release
Application : Coating Systems & Additives, Controlled & Modified Release
Excipient Details : Film Forming Agent, Wet/Dry Granulation- Binder,Thickening & Suspension Agent, Non-Gelatin Capsule Manufacturing & Enteric Film Coating Systems
Pharmacopoeia Ref : USP, EP, and JP
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Taste Masking
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Direct Compression, Fillers, Diluents & Binders, Taste Masking
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Direct Compression, Disintegrants & Superdisintegrants
Brand Name : Mannogem Granular
Application : Chewable & Orodispersible Aids, Direct Compression, Disintegrants & Superdisintegrants
Excipient Details : Mannogem Granular has larger particles, offering a softer texture for chewable tablets and excellent flow, disintegration, and compression properties.
Dosage Form : Tablet, Orodispersible Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Taste Masking
Application : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Taste Masking
Excipient Details : Advantol® 300 is a DC pharmaceutical excipient used to create soft chew or fast melt oral dosage forms through co-processing technology.
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Direct Compression, Taste Masking
Application : Chewable & Orodispersible Aids, Direct Compression, Taste Masking
Excipient Details : MS90 is a directly compressible magnesium hydroxide with starch used for chewable tablets due to its smooth mouthfeel and creamy texture.
Pharmacopoeia Ref : NA
Technical Specs : PSD D50: 150-170 µm, Tapped Density: 0.80
Ingredient(s) : Magnesium Hydroxide Excipient
Dosage Form : Tablet, Orodispersible Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Fillers, Diluents & Binders, Taste Masking
Application : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Fillers, Diluents & Binders, Taste Masking
Excipient Details : Compressol® SM, co-processed polyol, is a DC excipient of mannitol & sorbitol with superior compactibility, low disintegration time & hygroscopicity.
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Direct Compression, Fillers, Diluents & Binders, Taste Masking
Application : Chewable & Orodispersible Aids, Direct Compression, Fillers, Diluents & Binders, Taste Masking
Excipient Details : CS90 is a directly compressible calcium carbonate with starch used for chewable tablets due to its smooth mouthfeel and creamy texture.
Pharmacopoeia Ref : NA
Technical Specs : PSD D50: 150-175 µm, Tapped Density: 0.85
Ingredient(s) : Calcium Carbonate Excipient
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Direct Compression
Application : Direct Compression, Disintegrants & Superdisintegrants
Excipient Details : Mannogem 2080 exhibits excellent flow, disintegration and compression properties.
Dosage Form : Tablet
Grade : Oral
Category : Direct Compression, Fillers, Diluents & Binders, Taste Masking
Application : Direct Compression, Fillers, Diluents & Binders, Taste Masking
Excipient Details : CM90 is a directly compressible, granulated calcium carbonate with maltodextrin used for swallow tablets due to its high density and compressibility.
Pharmacopoeia Ref : NA
Technical Specs : PSD D50: 490- 500 µm; Tapped Density: 1.50
Ingredient(s) : Calcium Carbonate Excipient
Dosage Form : Tablet, Orodispersible Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Taste Masking
Application : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Taste Masking
Excipient Details : Advantol® 300 is a DC pharmaceutical excipient used to create soft chew or fast melt oral dosage forms through co-processing technology.
Application : Disintegrants & Superdisintegrants
Excipient Details : Solvostar (Sodium Starch Glycolate) is used as a superdisintegrant in oral solid dosage forms such as tablets and capsules.
Pharmacopoeia Ref : USP-NF, BP, IP, EP, DMF, EXCiP...
Technical Specs : Solvostar 2% to 8%
Ingredient(s) : Sodium Starch Glycolate
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders
Brand Name : Mannogem XL Mannitol
Application : Chewable & Orodispersible Aids, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders
Excipient Details : Mannogem® XL is a DC spray-dried mannitol used to enhance mannitol formulation tabletability & provide superior binding & quick disintegration.
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Direct Compression, Disintegrants & Superdisintegrants
Brand Name : Mannogem Granular
Application : Chewable & Orodispersible Aids, Direct Compression, Disintegrants & Superdisintegrants
Excipient Details : Mannogem Granular has larger particles, offering a softer texture for chewable tablets and excellent flow, disintegration, and compression properties.
Application : Direct Compression, Disintegrants & Superdisintegrants
Excipient Details : Mannogem 2080 exhibits excellent flow, disintegration and compression properties.
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Fillers, Diluents & Binders, Taste Masking
Dosage Form : Capsule, Granule / Pellet, Tablet, Injectable / Parenteral
Grade : Parenteral, Oral
Category : Fillers, Diluents & Binders, Thickeners and Stabilizers
Dosage Form : Cream / Lotion / Ointment, Gel, Injectable / Parenteral, Softgels
Grade : Parenteral, Oral, Topical
Category : Film Formers & Plasticizers, Parenteral, Thickeners and Stabilizers, Topical
Dosage Form : Injectable / Parenteral
Grade : Parenteral
Category : Parenteral, Thickeners and Stabilizers
Dosage Form : Injectable / Parenteral, Tablet
Grade : Parenteral, Oral
Category : Fillers, Diluents & Binders, Parenteral, Thickeners and Stabilizers
Dosage Form : Capsule, Granule / Pellet, Tablet, Injectable / Parenteral
Grade : Parenteral, Oral
Category : Fillers, Diluents & Binders, Thickeners and Stabilizers
Dosage Form : Capsule, Granule / Pellet, Tablet, Injectable / Parenteral
Grade : Parenteral, Oral
Category : Fillers, Diluents & Binders, Thickeners and Stabilizers
Dosage Form : Capsule, Granule / Pellet, Tablet, Injectable / Parenteral
Grade : Parenteral, Oral
Category : Fillers, Diluents & Binders, Thickeners and Stabilizers
Dosage Form : Suspension, Tablet
Grade : Oral
Category : Rheology Modifiers, Thickeners and Stabilizers
Dosage Form : Granule / Pellet, Tablet
Grade : Oral
Category : Fillers, Diluents & Binders, Granulation, Solubilizers
Application : Disintegrants & Superdisintegrants
Excipient Details : Crospovidone is used as a disintegrant in solid oral dosage forms such as tablets and capsules.
Dosage Form : Capsule, Tablet, Topical Film, Transdermal Patch
Grade : Not Available
Category : Controlled & Modified Release, Direct Compression, Granulation
Application : Controlled & Modified Release, Direct Compression, Granulation
Excipient Details : For non-erodible matrices using direct compression, Controlled release matrix. Matrix former in transdermal patches and topical films.
Pharmacopoeia Ref : Ph. Eur., USP-NF, JP-JPE: 80 %...
Technical Specs : Not Available
Ingredient(s) : Lauryl Sulfate
Dosage Form : Tablet
Grade : Oral
Category : Direct Compression, Fillers, Diluents & Binders, Granulation
Application : Direct Compression, Fillers, Diluents & Binders, Granulation
Excipient Details : AceCel is suitable for majority of the directly compressible actives, combines good flow and high compressibility.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose
Application : Granulation
Excipient Details : Crospovidone is used as a granulating agent in pharmaceutical formulations such as tablets, granules, and pellets.
Dosage Form : Tablet
Grade : Oral
Category : Direct Compression, Fillers, Diluents & Binders, Granulation
Application : Direct Compression, Fillers, Diluents & Binders, Granulation
Excipient Details : HiCel acts as a strong & dry binder. It facilitates low tablet friability & promotes rapid tablet disintegration.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose
Dosage Form : Granule / Pellet, Tablet
Grade : Not Available
Category : Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Granulation
Application : Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Granulation
Excipient Details : Tablets, Granules, Pills, Disintegrants and fillers.
Grade : Oral
Category : Disintegrants & Superdisintegrants, Granulation, Lubricants & Glidants
Application : Disintegrants & Superdisintegrants, Granulation, Lubricants & Glidants
Application : Fillers, Diluents & Binders, Granulation
Excipient Details : Fully Pregel Starch Corn is used as a binder in wet granulation in the manufacturing of tablets.
Pharmacopoeia Ref : USP/NF, Ph. Eur., Ch. P.
Technical Specs : NA
Ingredient(s) : Corn Starch, Pregelatinized
Application : Fillers, Diluents & Binders, Granulation
Pharmacopoeia Ref : Conforms to USP-NF, Ph.Eur., J...
Technical Specs : Density- Tapped density- 857 g/l, Bulk density- 589 g/l; Particle...
Ingredient(s) : Lactose Monohydrate
Dosage Form : Granule / Pellet, Tablet
Grade : Oral
Category : Fillers, Diluents & Binders, Granulation, Solubilizers
Dosage Form : Capsule, Granule / Pellet, Tablet
Grade : Oral
Category : Coating Systems & Additives, Controlled & Modified Release, Solubilizers
Brand Name : EUDRAGIT® L 100-55
Application : Coating Systems & Additives, Controlled & Modified Release, Solubilizers
Excipient Details : EUDRAGIT® L 100-55 (powder) is used in delayed release coatings to enhance solubility of poorly soluble drugs such as tablets, capsules & granules.
Pharmacopoeia Ref : NA
Technical Specs : NA
Ingredient(s) : Methacrylic Acid - Ethyl Acrylate Copolymer
Application : Solubilizers
Excipient Details : EUDRAGIT® L 12,5 (organic solution), a delayed release polymer is used to increase solubility of poorly soluble products such as tablets and capsules.
Pharmacopoeia Ref : NA
Technical Specs : NA
Ingredient(s) : Methacrylic Acid Methyl Methacrylate Copolymer
Application : Solubilizers
Excipient Details : EUDRAGIT® S 12,5 (Organic solution) a delayed release polymer, used to increase solubility of poorly soluble drugs such as tablets and capsules.
Pharmacopoeia Ref : NA
Technical Specs : NA
Ingredient(s) : Methacrylic Acid Methyl Methacrylate Copolymer
Dosage Form : Cream / Lotion / Ointment, Gel, Solution
Grade : Oral, Topical
Category : Emulsifying Agents, Solubilizers, Topical
Application : Emulsifying Agents, Solubilizers, Topical
Excipient Details : Hydrosol 50 is used as a solubilizer and emulsifying agent in oral and topical liquid and semi-solid dosage forms.
Pharmacopoeia Ref : USP/NF
Technical Specs : N/A
Ingredient(s) : Polyoxyl 40 Hydrogenated Castor Oil
Grade : Oral
Category : Coating Systems & Additives, Controlled & Modified Release, Solubilizers
Brand Name : Lumacril L-100D 55
Application : Coating Systems & Additives, Controlled & Modified Release, Solubilizers
Excipient Details : Lumacril L-100D 55 is an anionic copolymer used in delayed & sustained release coatings to enhance solubility for poorly soluble drugs in OSDs.
Pharmacopoeia Ref : USP-NF, Ph.Eur, BP, JP
Technical Specs : NA
Ingredient(s) : Methacrylic Acid - Ethyl Acrylate Copolymer
Brand Name : AFFINISOL HPMC HME
Application : Solubilizers
Excipient Details : Solubility enhancement, Spray-Dried Dispersion (SDD), Hot Melt Extrusion (HME)
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Fillers, Diluents & Binders, Taste Masking
Dosage Form : Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Taste Masking
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Direct Compression, Fillers, Diluents & Binders, Taste Masking
Dosage Form : Tablet, Orodispersible Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Taste Masking
Application : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Taste Masking
Excipient Details : Advantol® 300 is a DC pharmaceutical excipient used to create soft chew or fast melt oral dosage forms through co-processing technology.
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Taste Masking
Dosage Form : Tablet, Orodispersible Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Fillers, Diluents & Binders, Taste Masking
Application : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Fillers, Diluents & Binders, Taste Masking
Excipient Details : Compressol® SM, co-processed polyol, is a DC excipient of mannitol & sorbitol with superior compactibility, low disintegration time & hygroscopicity.
Application : Taste Masking, Thickeners and Stabilizers
Excipient Details : Maltodextrin is used as a stabilizing and taste masking agent in oral solid dosage forms such as tablets.
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders
Brand Name : Mannogem XL Mannitol
Application : Chewable & Orodispersible Aids, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders
Excipient Details : Mannogem® XL is a DC spray-dried mannitol used to enhance mannitol formulation tabletability & provide superior binding & quick disintegration.
Dosage Form : Orodispersible Tablet, Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Direct Compression
Grade : Oral
Category : Coating Systems & Additives, Film Formers & Plasticizers
Brand Name : EUDRAGIT® L 30 D-55
Application : Coating Systems & Additives, Film Formers & Plasticizers
Excipient Details : EUDRAGIT® L 30 D-55 (aqueous dispersion) is a delayed release polymer used as coating and film former in tablets, capsules, granules etc.
Pharmacopoeia Ref : NA
Technical Specs : NA
Ingredient(s) : Methacrylic Acid - Ethyl Acrylate Copolymer
Dosage Form : Cream / Lotion / Ointment, Gel, Injectable / Parenteral, Softgels
Grade : Parenteral, Oral, Topical
Category : Film Formers & Plasticizers, Parenteral, Thickeners and Stabilizers, Topical
Grade : Oral
Category : Film Formers & Plasticizers, Surfactant & Foaming Agents, Taste Masking
Dosage Form : Emulsion, Tablet
Grade : Oral, Ophthalmic
Category : Disintegrants & Superdisintegrants, Emulsifying Agents, Film Formers & Plasticizers, Thickeners and Stabilizers
Brand Name : Hydroxypropyl Methyl Cellulose
Application : Disintegrants & Superdisintegrants, Emulsifying Agents, Film Formers & Plasticizers, Thickeners and Stabilizers
Excipient Details : Hydroxypropyl Methyl Cellulose is used as a film-former, disintegrant, thickener, and emulsifier in tablets, emulsions, and ophthalmic formulations.
Brand Name : Hydroxypropyl Methyl Cellulose
Application : Coating Systems & Additives
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Grade : Not Available
Category : Coating Systems & Additives, Film Formers & Plasticizers
Brand Name : ReadiLYCOAT® D CLEAR 110.01
Application : Coating Systems & Additives, Film Formers & Plasticizers
Excipient Details : A natural inert polymer and ready-to-use coating system for fast aqueous film coating saves up to 50% or more time.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Pea Starch, Sorbitol, Stearic Acid
Dosage Form : Capsule, Cream / Lotion / Ointment
Grade : Topical and Oral
Category : Film Formers & Plasticizers, Topical
Application : Film Formers & Plasticizers, Topical
Dosage Form : Tablet
Grade : Oral
Category : Coating Systems & Additives, Film Formers & Plasticizers
Application : Coating Systems & Additives, Film Formers & Plasticizers
Grade : Not Available
Category : Coating Systems & Additives, Film Formers & Plasticizers
Brand Name : ReadiLYCOAT® D CLEAR 110.01 MS
Application : Coating Systems & Additives, Film Formers & Plasticizers
Excipient Details : A natural inert polymer and ready-to-use coating system for fast aqueous film coating saves up to 50% or more time.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Pea Starch, Sorbitol, Stearic Acid
Grade : Oral
Category : Fillers, Diluents & Binders, Lubricants & Glidants
Application : Fillers, Diluents & Binders, Lubricants & Glidants
Excipient Details : Most popular excipient for the production of tablets and capsules. Offering an efficient and low dosage in capsules.
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Specific Surface Area-6-10 m2/g; Particle Size-7-11 µm
Ingredient(s) : Magnesium Stearate
Dosage Form : Granule / Pellet, Tablet
Grade : Oral & Topical
Category : Controlled & Modified Release, Lubricants & Glidants
Application : Controlled & Modified Release, Lubricants & Glidants
Excipient Details : Talc is a widely used as a dissolution retardant in the development of controlled release products. Talc is also used as a lubricant in tablet formulations.
Brand Name : Magnesium Stearate
Application : Lubricants & Glidants
Excipient Details : Magnesium stearate is the magnesium salt of stearic acid. It acts as a lubricating agent in tablet manufacturing.
Pharmacopoeia Ref : USP/BP/EP/PH.EUR
Technical Specs : Not Available
Ingredient(s) : Magnesium Stearate
Grade : Oral
Category : Fillers, Diluents & Binders, Lubricants & Glidants
Application : Fillers, Diluents & Binders, Lubricants & Glidants
Excipient Details : Higher specific surface area and a smaller median particle size. This product is preferred for more critical and very fine herbal formulations.
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Specific Surface Area-8-12 m2/g; Particle Size-5-9 µm
Ingredient(s) : Magnesium Stearate
Grade : Oral
Category : Disintegrants & Superdisintegrants, Granulation, Lubricants & Glidants
Application : Disintegrants & Superdisintegrants, Granulation, Lubricants & Glidants
Brand Name : Magnesium Stearate
Application : Lubricants & Glidants
Excipient Details : Lubricants, Anti-adhesive, Glidant
Grade : Oral
Category : Fillers, Diluents & Binders, Lubricants & Glidants
Application : Fillers, Diluents & Binders, Lubricants & Glidants
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Specific Surface Area-6-8 m2/g; Particle Size-7-11 µm
Ingredient(s) : Magnesium Stearate
Application : Fillers, Diluents & Binders, Taste Masking
Excipient Details : Mannitol is used as a filler, bulking agent and taste masking agent in ODT formulations such as tablets.
Dosage Form : Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Taste Masking
Application : Co-Processed Excipients
Excipient Details : Mannitol is a co-processed excipient with superior compactibility, lower disintegration time, and hygroscopicity.
Grade : Oral
Category : Co-Processed Excipients, Fillers, Diluents & Binders
Application : Co-Processed Excipients, Fillers, Diluents & Binders
Excipient Details : ProBlend (SMCC) is a co-processed excipient consists of microcrystalline cellulose & colloidal silicon dioxide, used as a diluent & binder in OSDs.
Pharmacopoeia Ref : USP-NF, DMF, EXCiPAT, KOSHER, ...
Technical Specs : NA
Ingredient(s) : Silicified Microcrystalline Cellulose
Dosage Form : Tablet, Orodispersible Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Taste Masking
Application : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Disintegrants & Superdisintegrants, Fillers, Diluents & Binders, Taste Masking
Excipient Details : Advantol® 300 is a DC pharmaceutical excipient used to create soft chew or fast melt oral dosage forms through co-processing technology.
Application : Co-Processed Excipients, Fillers, Diluents & Binders
Excipient Details : Microlose (Lactose Monohydrate & Microcrystalline Cellulose) is used as a diluent in oral dosage forms such as tablets.
Pharmacopoeia Ref : DMF, EXCiPAT, KOSHER, HALAL, W...
Technical Specs : Lactose Monohydrate – 40%, Microcrystalline cellulose – 60%
Ingredient(s) : Lactose Monohydrate
Dosage Form : Tablet, Orodispersible Tablet
Grade : Oral
Category : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Fillers, Diluents & Binders, Taste Masking
Application : Chewable & Orodispersible Aids, Co-Processed Excipients, Direct Compression, Fillers, Diluents & Binders, Taste Masking
Excipient Details : Compressol® SM, co-processed polyol, is a DC excipient of mannitol & sorbitol with superior compactibility, low disintegration time & hygroscopicity.
Dosage Form : Injectable / Parenteral, Tablet
Grade : Not Available
Category : Fillers, Diluents & Binders, Parenteral
Application : Fillers, Diluents & Binders, Parenteral
Excipient Details : It is used in producing the injection solution, reducing the encephalic pressure, the intraocular pressure etc.
Pharmacopoeia Ref : CP/BP/ EP/ USP/IP
Technical Specs : Pharma Grade/ Medicine Grade/Injectable Grade
Ingredient(s) : Mannitol
Dosage Form : Cream / Lotion / Ointment, Gel, Injectable / Parenteral, Softgel Capsule, Softgels
Grade : Parenteral, Topical, Oral
Category : Fillers, Diluents & Binders, Film Formers & Plasticizers, Parenteral, Surfactant & Foaming Agents, Thickeners and Stabilizers, Topical
Brand Name : Polyethylene Glycol 400
Application : Fillers, Diluents & Binders, Film Formers & Plasticizers, Parenteral, Surfactant & Foaming Agents, Thickeners and Stabilizers, Topical
Excipient Details : PEG 400 is used as a suspending agent, stabilizer, plasticizer and filler in OSDs, liquids & semi-solids and as a solvent for parenteral formulations.
Dosage Form : Cream / Lotion / Ointment, Gel, Injectable / Parenteral, Softgels
Grade : Parenteral, Oral, Topical
Category : Film Formers & Plasticizers, Parenteral, Thickeners and Stabilizers, Topical
Dosage Form : Cream / Lotion / Ointment, Gel, Solution
Grade : Oral, Topical
Category : Emulsifying Agents, Solubilizers, Topical
Application : Emulsifying Agents, Solubilizers, Topical
Excipient Details : Hydrosol 50 is used as a solubilizer and emulsifying agent in oral and topical liquid and semi-solid dosage forms.
Pharmacopoeia Ref : USP/NF
Technical Specs : N/A
Ingredient(s) : Polyoxyl 40 Hydrogenated Castor Oil
Dosage Form : Capsule, Cream / Lotion / Ointment
Grade : Topical and Oral
Category : Film Formers & Plasticizers, Topical
Application : Film Formers & Plasticizers, Topical
Dosage Form : Capsule, Cream / Lotion / Ointment, Gel, Tablet
Grade : Topical and Oral
Category : Controlled & Modified Release, Topical
Brand Name : Polyethylene Glycol 400
Application : Controlled & Modified Release, Topical
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : polyethylene glycol
Brand Name : Polyethylene Glycol 200 NF
Application : Topical
Excipient Details : A & C's Polyethylene Glycol 200 is an excipient which meets the NF monograph.
Pharmacopoeia Ref : NF
Technical Specs : Polyethylene Glycol 200; Polyethylene Glycol 300; Polyethylene Gl...
Ingredient(s) : polyethylene glycol
Dosage Form : Cream / Lotion / Ointment, Emulsion, Gel, Suppository
Grade : Not Available
Category : Thickeners and Stabilizers, Topical
Brand Name : Kollisolv PEG 8000
Application : Thickeners and Stabilizers, Topical
Excipient Details : Forms anhydrous, hydrophilic ointments in combination with low mol. weight PEG
Brand Name : Polyethylene Glycol 300 NF
Application : Topical
Excipient Details : A & C's Polyethylene Glycol 300 is an excipient which meets the NF monograph.
Brand Name : Polyethylene Glycol 400
Application : Topical
Excipient Details : A&C's Polyethylene Glycol 400 USP-NF is a short chain non-ionic surfactant.
Pharmacopoeia Ref : Not Available
Technical Specs : Low Endotoxin
Ingredient(s) : polyethylene glycol
Dosage Form : Capsule, Emulsion, Softgel Capsule, Solution, Tablet, Topical Film
Grade : Not Available
Category : Film Formers & Plasticizers, Solubilizers, Topical
Application : Film Formers & Plasticizers, Solubilizers, Topical
Excipient Details : Liquid plasticizer with high ADI, hydrophilic solvent & humectant in emulsions, skin penetration enhancer in topical formulaitons.
Pharmacopoeia Ref : Ph. Eur., JP, FCC, USP
Technical Specs : Not Available
Ingredient(s) : Propylene Glycol
Application : Empty Capsules
Excipient Details : Quali-V®-I is the first plant-based capsule that responds to the particular functional properties required for use in dry powder inhaler (DPI) devices.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Application : Empty Capsules
Excipient Details : ACGCAPS™ GL / HL is available in gelatin and cellulose (HPMC) material options and can be used for liquids, semi-liquids, hot-melts and combination fills.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Gelatin, Unspecified
Application : Empty Capsules
Excipient Details : ACGCAPS™ GR can fulfill immediate release needs and is available in 15 sizes (from # 000 to # 5).
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Gelatin, Unspecified
Application : Empty Capsules
Excipient Details : Quali-G™ is first preservative-free gelatin capsule, the market standard solid dosage form designed to meet the demanding requirements of the pharmaceutical industry.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Gelatin, Unspecified
Application : Empty Capsules
Excipient Details : ACGCAPS™ GI / HI is available in gelatin and cellulose (HPMC) material options and is designed for optimal performance with DPI formulations and various inhalation devices.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Gelatin, Unspecified
Application : Empty Capsules
Excipient Details : ACGCAPS™ GC is specially designed for use in clinical trials and comes in the available sizes: AA, A & B.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Gelatin, Unspecified
Application : Empty Capsules
Excipient Details : DBcaps® capsules are developed with a tamper-evident design to specifically address the clinical trial challenges of testing without bias.
Pharmacopoeia Ref : Not Available
Technical Specs : Size # AAA- E
Ingredient(s) : Gelatin, Unspecified
Application : Empty Capsules
Pharmacopoeia Ref : Certified Vegan, Non-GMO, Vege...
Technical Specs : Size # 00el - 4
Ingredient(s) : Gelatin, Unspecified
Application : Empty Capsules
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Gelatin, Unspecified
Application : Fillers, Diluents & Binders, Taste Masking
Excipient Details : Mannitol is used as a filler, bulking agent and taste masking agent in ODT formulations such as tablets.
Dosage Form : Cream / Lotion / Ointment, Gel, Injectable / Parenteral, Softgels
Grade : Parenteral, Oral, Topical
Category : Film Formers & Plasticizers, Parenteral, Thickeners and Stabilizers, Topical
Dosage Form : Injectable / Parenteral
Grade : Parenteral
Category : Parenteral, Thickeners and Stabilizers
Application : Co-Processed Excipients
Excipient Details : Mannitol is a co-processed excipient with superior compactibility, lower disintegration time, and hygroscopicity.
Dosage Form : Injectable / Parenteral, Tablet
Grade : Parenteral, Oral
Category : Fillers, Diluents & Binders, Parenteral, Thickeners and Stabilizers
Brand Name : Lactose Monohydrate
Application : Parenteral
Excipient Details : Lactose monohydrate is used as a diluent in inhalation and lyophilized preparations.
Dosage Form : Capsule, Injectable / Parenteral, Tablet
Grade : Oral, Parenteral
Category : Fillers, Diluents & Binders, Parenteral
Dosage Form : Capsule, Cream / Lotion / Ointment
Grade : Topical and Oral
Category : Film Formers & Plasticizers, Topical
Application : Film Formers & Plasticizers, Topical
Dosage Form : Cream / Lotion / Ointment, Emulsion, Gel, Solution, Tablet
Grade : Topical, Oral
Category : API Stability Enhancers, Emulsifying Agents, Fillers, Diluents & Binders
Application : Emulsifying Agents
Excipient Details : HDK N20 Pharma is used as a pharmaceutical emulsifying agent in tablets, capsules, syrups, and solutions.
Dosage Form : Emulsion, Tablet
Grade : Oral, Ophthalmic
Category : Disintegrants & Superdisintegrants, Emulsifying Agents, Film Formers & Plasticizers, Thickeners and Stabilizers
Brand Name : Hydroxypropyl Methyl Cellulose
Application : Disintegrants & Superdisintegrants, Emulsifying Agents, Film Formers & Plasticizers, Thickeners and Stabilizers
Excipient Details : Hydroxypropyl Methyl Cellulose is used as a film-former, disintegrant, thickener, and emulsifier in tablets, emulsions, and ophthalmic formulations.
Brand Name : Hydroxypropyl Methyl Cellulose
Application : Coating Systems & Additives
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Cream / Lotion / Ointment, Gel, Solution
Grade : Oral, Topical
Category : Emulsifying Agents, Solubilizers, Topical
Application : Emulsifying Agents, Solubilizers, Topical
Excipient Details : Hydrosol 50 is used as a solubilizer and emulsifying agent in oral and topical liquid and semi-solid dosage forms.
Pharmacopoeia Ref : USP/NF
Technical Specs : N/A
Ingredient(s) : Polyoxyl 40 Hydrogenated Castor Oil
Application : Emulsifying Agents, Lubricants & Glidants
Excipient Details : Glidant; Emulsion Stabilizer; Anti-caking Agent.
Dosage Form : Cream / Lotion / Ointment, Tablet
Grade : Oral
Category : Emulsifying Agents, Lubricants & Glidants, Thickeners and Stabilizers
Application : Emulsifying Agents, Lubricants & Glidants, Thickeners and Stabilizers
Dosage Form : Cream / Lotion / Ointment, Emulsion, Gel, Solution, Tablet
Grade : Topical, Oral
Category : API Stability Enhancers, Emulsifying Agents, Fillers, Diluents & Binders
Application : API Stability Enhancers, Thickeners and Stabilizers
Excipient Details : Adsorbent, Moisture Protection, Stabilization of API
Pharmacopoeia Ref : USP-NF, JP, EP
Technical Specs : Also Available as FUJISIL-F
Ingredient(s) : Silicon Dioxide
Grade : Not Available
Category : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Application : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Excipient Details : Controlled Release, Direct Compression,Wet Granulation,Tablet Coating, Liquid Solutions and Suspensions
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Capsule
Grade : Oral and Inhalation
Category : API Stability Enhancers, Vegetarian Capsules
Application : API Stability Enhancers, Vegetarian Capsules
Pharmacopoeia Ref : Certified Vegan, Non-GMO, Vege...
Technical Specs : "Water content – less than 9%, can be customized; Size # 00el -...
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Capsule
Grade : Oral
Category : API Stability Enhancers, Taste Masking, Vegetarian Capsules
Application : API Stability Enhancers, Taste Masking, Vegetarian Capsules
Pharmacopoeia Ref : Certified Vegan, Non-GMO, Vege...
Technical Specs : Size # 0-1
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Dosage Form : Capsule, Granule / Pellet, Tablet
Grade : Oral
Category : Coating Systems & Additives, Coloring Agents
Brand Name : Titanium dioxide PRETIOX AV01FG
Application : Coating Systems & Additives, Coloring Agents
Excipient Details : Titanium dioxide Pretiox AV01FG is used as a coloring and coating agent in oral solid dosage forms such as capsules, tablets, granules, and pellets.
Pharmacopoeia Ref : Fami-QS, Kosher, Halal, OHSAS ...
Technical Specs : Ti 59.95% and O 40.05%
Ingredient(s) : Titanium Dioxide
Dosage Form : Capsule, Granule / Pellet, Tablet
Grade : Oral
Category : Coating Systems & Additives, Coloring Agents
Dosage Form : Granule / Pellet, Tablet
Grade : Oral
Category : Coating Systems & Additives, Coloring Agents
Brand Name : SEPIFILM Classic Range
Application : Coating Systems & Additives, Coloring Agents
Excipient Details : Coating
Dosage Form : Granule / Pellet, Tablet
Grade : Oral
Category : Coating Systems & Additives, Coloring Agents
Brand Name : SEPIFILM PW Range
Application : Coating Systems & Additives, Coloring Agents
Excipient Details : Coating
Grade : Not Available
Category : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Application : API Stability Enhancers, Coating Systems & Additives, Controlled & Modified Release, Direct Compression, Granulation, Rheology Modifiers, Vegetarian Capsules
Excipient Details : Controlled Release, Direct Compression,Wet Granulation,Tablet Coating, Liquid Solutions and Suspensions
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Hydroxypropyl Methylcellulose
Dosage Form : Capsule
Grade : Oral and Inhalation
Category : API Stability Enhancers, Vegetarian Capsules
Application : API Stability Enhancers, Vegetarian Capsules
Pharmacopoeia Ref : Certified Vegan, Non-GMO, Vege...
Technical Specs : "Water content – less than 9%, can be customized; Size # 00el -...
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Application : Controlled & Modified Release, Vegetarian Capsules
Pharmacopoeia Ref : Complies with relevant Europea...
Technical Specs : Water content – less than 6%; Size #0
Ingredient(s) : HPMC AS
Dosage Form : Capsule
Grade : Oral
Category : API Stability Enhancers, Taste Masking, Vegetarian Capsules
Application : API Stability Enhancers, Taste Masking, Vegetarian Capsules
Pharmacopoeia Ref : Certified Vegan, Non-GMO, Vege...
Technical Specs : Size # 0-1
Ingredient(s) : Hydroxypropyl Methyl Cellulose
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
58
PharmaCompass offers a list of Dextroamphetamine Saccharate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Dextroamphetamine Saccharate manufacturer or Dextroamphetamine Saccharate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Dextroamphetamine Saccharate manufacturer or Dextroamphetamine Saccharate supplier.
PharmaCompass also assists you with knowing the Dextroamphetamine Saccharate API Price utilized in the formulation of products. Dextroamphetamine Saccharate API Price is not always fixed or binding as the Dextroamphetamine Saccharate Price is obtained through a variety of data sources. The Dextroamphetamine Saccharate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2, including repackagers and relabelers. The FDA regulates AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 supplier is an individual or a company that provides AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 active pharmaceutical ingredient (API) or AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 finished formulations upon request. The AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 suppliers may include AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 API manufacturers, exporters, distributors and traders.
click here to find a list of AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 DMF (Drug Master File) is a document detailing the whole manufacturing process of AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 active pharmaceutical ingredient (API) in detail. Different forms of AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 DMFs exist exist since differing nations have different regulations, such as AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 DMF submitted to regulatory agencies in the US is known as a USDMF. AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 USDMF includes data on AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 suppliers with NDC on PharmaCompass.
AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 GMP manufacturer or AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 GMP API supplier for your needs.
A AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 CoA (Certificate of Analysis) is a formal document that attests to AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2's compliance with AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 specifications and serves as a tool for batch-level quality control.
AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 CoA mostly includes findings from lab analyses of a specific batch. For each AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 may be tested according to a variety of international standards, such as European Pharmacopoeia (AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 EP), AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (AMPHETAMINE ASP AMPHETAMINE SULF DEXTROAMPHET SACCHARATE & DEXTR SULF-2 USP).
