



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
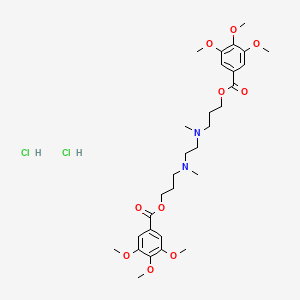

1. Andiamine
2. Ditrimine
3. Hexobendine
4. Ustimon
1. Andiamine
2. 50-62-4
3. Reoxyl
4. Ustimon
5. P60cs4tihv
6. Flussicor
7. 3-[methyl-[2-[methyl-[3-(3,4,5-trimethoxybenzoyl)oxypropyl]amino]ethyl]amino]propyl 3,4,5-trimethoxybenzoate;dihydrochloride
8. Hexobendine Hcl
9. (ethane-1,2-diylbis(methylazanediyl))bis(propane-3,1-diyl) Bis(3,4,5-trimethoxybenzoate) Dihydrochloride
10. 3-[methyl-[2-[methyl-[3-(3,4,5-trimethoxybenzoyl)oxypropyl]amino]ethyl]amino]propyl3,4,5-trimethoxybenzoate Dihydrochloride
11. Einecs 200-054-7
12. Unii-p60cs4tihv
13. Hexobendine Hydrochloride
14. Hexobendine Dihy-drochloride
15. Benzoic Acid, 3,4,5-trimethoxy-, 1,2-ethanediylbis((methylimino)-3,1-propanediyl) Ester, 2hcl
16. N,n'-dimethyl-n,n'-bis(3-(3',4',5'-trimethoxybenzoxy)propyl)ethylenediamine Dihydrochloride
17. Schembl317378
18. Dtxsid90964485
19. St-7090
20. Hexobendine Dihydrochloride [mi]
21. Benzoic Acid, 3,4,5-trimethoxy-, Diester With 3,3'-(ethylenebis(methylimino))di-1-propanol, Dihydrochloride
22. Hexobendine Hydrochloride [who-dd]
23. Ft-0669197
24. Q27286267
25. (ethane-1,2-diyl)bis[(methylazanediyl)propane-3,1-diyl] Bis(3,4,5-trimethoxybenzoate)--hydrogen Chloride (1/2)
| Molecular Weight | 665.6 g/mol |
|---|---|
| Molecular Formula | C30H46Cl2N2O10 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 21 |
| Exact Mass | 664.2529511 g/mol |
| Monoisotopic Mass | 664.2529511 g/mol |
| Topological Polar Surface Area | 115 Ų |
| Heavy Atom Count | 44 |
| Formal Charge | 0 |
| Complexity | 684 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)


