
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
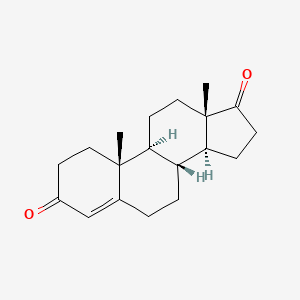

1. 4 Androstene 3,17 Dione
2. 4-androstene-3,17-dione
3. Delta 4 Androstenedione
4. Delta-4-androstenedione
1. 4-androstene-3,17-dione
2. Androst-4-ene-3,17-dione
3. 63-05-8
4. 4-androstenedione
5. Androtex
6. 3,17-dioxoandrost-4-ene
7. Delta-4-androstenedione
8. Fecundin
9. Skf 2170
10. Androstendione
11. Delta-4-androstene-3,17-dione
12. 4-androstene-3-17-dione
13. Delta-4-androsten-3,17-dione
14. 4-androsten-3,17-dione
15. Androstenedione [jan]
16. Nsc 9563
17. Delta-(sup4)-androsten-3,17-dione
18. Delta(sup 4)-androstene-3,17-dione
19. 17-ketotestosterone
20. (8r,9s,10r,13s,14s)-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1h-cyclopenta[a]phenanthrene-3,17-dione
21. Chebi:16422
22. Mls000028510
23. Delta(4)-androsten-3,17-dione
24. Nsc9563
25. 409j2j96vr
26. Delta(4)-androstene-3,17-dione
27. Nsc-9563
28. .delta.4-androstene-3,17-dione
29. Androstenedione (jan)
30. Ncgc00023902-06
31. Smr000058281
32. Dsstox_cid_4523
33. Dsstox_rid_77443
34. Dsstox_gsid_24523
35. Cas-63-05-8
36. (8r,9s,10r,13s,14s)-10,13-dimethyl-7,8,9,10,11,12,13,14,15,16-decahydro-1h-cyclopenta[a]phenanthrene-3,17(2h,6h)-dione
37. Androst-4-ene-3,17-dione (androstenedione)
38. Hsdb 7335
39. Einecs 200-554-5
40. Chembl274826
41. Adione
42. Unii-409j2j96vr
43. Delta4-androstene-3,17-dione
44. Delta4-androstenedione
45. 4-andendion
46. Androsten-3,17-dione
47. Opera_id_1694
48. Spectrum5_002059
49. [4-14c]-androstenedione
50. Epitope Id:135869
51. Ec 200-554-5
52. Testosterone Ep Impurity A
53. Androst-4-en-3,17-dione
54. Androstenedione [mi]
55. Bidd:pxr0101
56. D4-androstene-3,17-dione
57. Lopac0_000114
58. Schembl23272
59. Mls000563086
60. Mls002152886
61. Androstenedione [hsdb]
62. (4)-androsten-3,17-dione
63. Androstenedione [vandf]
64. Gtpl2860
65. Androstenedione [mart.]
66. Androstenedione [who-dd]
67. Dtxsid8024523
68. Bdbm91713
69. .delta.4-androsten-3,17-dione
70. Androst-4-en-3,17-dione, 2
71. Hms2231f18
72. Zinc4428526
73. Tox21_110893
74. Tox21_202269
75. Tox21_300579
76. Bbl033517
77. C1015
78. Lmst02020007
79. Stk801871
80. Akos005622710
81. Tox21_110893_1
82. [4-14c]androst-4-ene-3,17-dione
83. Ccg-204209
84. Db01536
85. .delta.-(sup4)-androsten-3,17-dione
86. Estr-5-ene-3,17-diol,(3b,17b)-
87. Ncgc00023902-03
88. Ncgc00023902-04
89. Ncgc00023902-05
90. Ncgc00023902-07
91. Ncgc00023902-08
92. Ncgc00023902-09
93. Ncgc00254238-01
94. Ncgc00259818-01
95. Ac-11042
96. Ac-33197
97. Androstenedione; 3,17-dioxo-4-andostene
98. Vs-12118
99. A0845
100. Eu-0100114
101. Ft-0657562
102. Testosterone Impurity A [ep Impurity]
103. Androstenedione (androst-4-ene-3,17-dione)
104. Wln: L E5 B666 Fv Ov Mutj A1 E1
105. A 9630
106. C00280
107. D00051
108. Testosterone Related Compound A [usp-rs]
109. Q411064
110. Sr-01000003096
111. Sr-01000075697
112. Sr-01000003096-4
113. Sr-01000075697-1
114. W-104936
115. 4-androstene-3,17-dione 100 Microg/ml In Methanol/water
116. 8f5f4dcb-1164-4f2c-b4e3-3b74f684b189
117. 4-androstene-3,17-dione, Vetranal(tm), Analytical Standard
118. Androstenedione (androst-4-ene-3,17-dione) 1.0 Mg/ml In Acetonitrile
119. (3as,3br,9ar,9bs,11as)-9a,11a-dimethyl-1h,2h,3h,3ah,3bh,4h,5h,7h,8h,9h,9ah,9bh,10h,11h,11ah-cyclopenta[a]phenanthrene-1,7-dione
120. (8r,10r,13s)-10,13-dimethyl-1,6,7,8,9,10,11,12,13,14,15,16-dodecahydro-2h-cyclopenta[a]phenanthrene-3,17-dione
121. 117598-81-9
122. Androst-4-ene-3,17-dione; 17-ketotestosterone; Androstenedione; 4-androstene-3,17-dione; 4-androstenedione
| Molecular Weight | 286.4 g/mol |
|---|---|
| Molecular Formula | C19H26O2 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 286.193280068 g/mol |
| Monoisotopic Mass | 286.193280068 g/mol |
| Topological Polar Surface Area | 34.1 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 546 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Androstenedione is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of April 21, 2017: https://clinicaltrials.gov/
Androstenedione and related molecules, if given in sufficient quantities and for sufficient duration, are likely to cause androgenic (and thus anabolic) or estrogenic effects in humans. ... Children and adolescents are particularly vulnerable to irreversible effects of androstenedione via its conversion to active sex steroids. These effects include disruption of normal sexual development, specifically virilization in girls associated with severe acne, excessive body and facial hair, deepening of the voice, permanent enlargement of the clitoris, disruption of the menstrual cycle, and infertility. The conversion to estrogens can cause feminization of boys, with breast enlargement and testicular atrophy. In girls, exposure to excess estrogens may confer long-term increased risk for breast and uterine cancer. Finally, in boys and girls, the combined effects of excessive androgens and estrogens can induce premature puberty, early closure of the growth plates of long bones, resulting in significant compromise of adult stature.
US FDA; FDA White Paper Health Effects of Androstenedione. March 11, 2004.
No data are available on the long-term safety of taking supplemental androstenedione. Adverse effects of exogenous testosterone in men include acne, testicular atrophy, gynecomastia, behavioral changes and possibly an increased risk of prostate cancer. Adverse effects of exogenous testosterone in women include hirsutism, deepening of the voice, acne, clitoral hypertrophy, amenorrhea, male-pattern baldness and coarsening of the skin. In adolescents, exogenous testosterone can lead to early closing of bone growth plates and decreased adult height. Other adverse effects of testosterone include hepatic failure and increased platelet aggregation. /Testosterone/
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 36
Androstenedione is contraindicated in those with prostate, breast and uterine cancer.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 36
Oral androstenedione has been found to decrease high-density lipoproteins (HDL)-cholesterol levels, which may increase risk of cardiovascular disease.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 36
For more Drug Warnings (Complete) data for Androstenedione (21 total), please visit the HSDB record page.
Absorption of /orally administered androstenedione/ appears variable, but some absorption does occur. Androstenedione is distributed to various tissues of the body ... .
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 36
/MILK/ It is not known whether anabolic steroids are distributed into breast milk. Problems in humans have not been documented. Women who take anabolic steroids should not breast feed. /Anabolic Steroids/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 141
A comparative study was performed to assess the metabolism of the androgen precursor androstenedione (AD) in two gastropod species from the Muricidae family: Bolinus brandaris and Hexaplex trunculus. AD was mainly converted to 5alpha-dihydrotestosterone by microsomal fractions isolated from Bolinus brandaris, whereas it was primarily metabolized to testosterone by Hexaplex trunculus. Sex differences in the metabolism of AD were only detected in Bolinus brandaris and attributed to higher 5alpha-reductase activity in males. Thereafter, the effect of the organotin compounds, tributyltin (TBT) and triphenyltin (TPT), on the metabolism of AD was investigated. A significant interference was only detected in females, and differences between the modes of action of the two compounds were observed: TPT was a strong inhibitor of 5alpha-reductase activity in B. brandaris at a concentration as low as 100 nM whereas only TBT (10 uM) altered the metabolism of AD in H. trunculus by increasing the activity 17beta-hydroxysteroid dehydrogenase (17beta-HSD). Thus, this work shows that the metabolism of the androgen precursor AD strongly differs among gastropod species, both in terms of activity and metabolic profile, and further demonstrates the ability of TBT and TPT to interfere with key enzymatic pathways involved in androgen synthesis.
PMID:18849009 Lyssimachou A et al; Comp Biochem Physiol C Toxicol Pharmacol 149 (3): 409-13 (2009)
Bone is a target organ of androgens. The mechanism by which these steroids exert their action within bone cells is still poorly understood. The metabolism of androstenedione, the major circulating androgen in women, was, therefore, assessed in osteoblast-like bone cells cultured from bone of 16 postmenopausal women (mean age, 69 yr; range, 56-80) and 3 elderly men (mean age, 71 yr; range, 69-73) undergoing total hip replacement. Each cell strain was incubated under standardized conditions with varying concentrations of [1,2,6,7- (3)H]androstenedione (0.05-5 uM). In every instance 5 alpha-reduced metabolites and 17 beta-hydroxysteroids were formed. There was no correlation between the volumetric density of the resected bone and androstenedione metabolism of the corresponding cultured bone cell strains. The apparent Km for the 5 alpha-reductase activity (sum of androstanedione and dihydrotestosterone) of all 19 cell strains was 0.7 +/- 0.1 uM (mean +/- SEM), and the apparent Km for 17 beta- hydroxysteroid dehydrogenase (sum of testosterone and dihydrotestosterone) was 2.3 +/- 0.8 uM (mean +/- SEM), values similar to those reported for other androgen target organs. Our results demonstrate that human osteoblast-like cells have the capacity to transform androstenedione into the more potent biological androgens testosterone and dihydrotestosterone. Since the Km values of both 5 alpha-reductase and 17 beta-hydroxysteroid dehydrogenase exceed the serum androstenedione concentration, the formation of testosterone and dihydrotestosterone appears to be mainly a function of substrate availability.
Bruch HR et al; Journal of Clinical Endocrinology & Metabolism 75: 101-105 (1992)
Up-regulation of aromatase expression in endometrial cells disseminated into the peritoneal cavity may enhance their survival via local estrogen synthesis, which may lead to endometriosis. The factors that mediate induction of aromatase in the endometrium are not well defined, but increased expression of steroidogenic factor (SF)-1 may play a role. The objective of the study was to determine whether androstenedione (A4), the predominant sex steroid in peritoneal fluid, regulates endometrial aromatase expression. This was a cell/tissue culture study ... conducted at an academic center. Quantitative real-time PCR, HPLC, and chromatin immunoprecipitation were used in this study. Treatment of cultured human endometrial explants and stromal cells with A4 (10 nm) significantly up-regulated expression of aromatase mRNA transcripts containing exon IIa at their 5'-ends. In endometrial stromal cells and the human endometrial surface epithelial (HES) cell line, induction of aromatase mRNA by A4 was associated with increased expression of SF-1. In HES cells, tritiated A4 was metabolized to estradiol, testosterone (T), dihydrotestosterone, and androstanediol. Both estradiol and T, but not nonaromatizable androgens, up-regulated aromatase and SF-1 mRNA in HES cells. Chromatin immunoprecipitation revealed that A4 enhanced recruitment of SF-1 to its response element (-136 bp) upstream of CYP19 exon IIa. This, together with the findings that both estrogen receptor antagonist, ICI 182,780, and aromatase inhibitor, fadrozole, suppressed A4 and T induction of aromatase and SF-1 mRNA, indicates that the inductive effects of A4 and T are mediated by their conversion to estrogens. Exposure of endometrial cells to A4 may enhance CYP19 gene expression through its aromatization to estrogens.
PMID:18559914 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2567859 Bukulmez O et al; J Clin Endocrinol Metab 93 (9): 3471-7 (2008)
Androstenedione is synthesized in the adrenal gland and gonads from dehydroepiandrosterone. It is metabolized by the enzyme 17 beta-hydroxy steroid dehydrogenase to testosterone, and by the aromatase enzyme complex to estrone. Estrone is metabolized to estradiol.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 34-5
Androstenedione is distributed to various tissues of the body and is metabolized to testosterone and estrone. The amount of testosterone produced per given dose of androstenedione appears to vary. Typically, a greater increase in serum testosterone is found in women compared to men, following intake of oral androstenedione.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 35
Androstenedione is a known human metabolite of testosterone.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
4-androstenedione is a 19-carbon steroid hormone produced in the adrenal glands and the gonads as an intermediate step in the biochemical pathway that produces the androgen testosterone and the estrogens estrone and estradiol.
Anabolic steroids reverses catabolic processes and negative nitrogen balance by promoting protein anabolism and stimulating appetite if there is concurrently a proper intake of calories and proteins. /Anabolic Steroids/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 141

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
ANALYTICAL

ABOUT THIS PAGE
71
PharmaCompass offers a list of Androstenedione API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Androstenedione manufacturer or Androstenedione supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Androstenedione manufacturer or Androstenedione supplier.
PharmaCompass also assists you with knowing the Androstenedione API Price utilized in the formulation of products. Androstenedione API Price is not always fixed or binding as the Androstenedione Price is obtained through a variety of data sources. The Androstenedione Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Androstenedione manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Androstenedione, including repackagers and relabelers. The FDA regulates Androstenedione manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Androstenedione API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Androstenedione supplier is an individual or a company that provides Androstenedione active pharmaceutical ingredient (API) or Androstenedione finished formulations upon request. The Androstenedione suppliers may include Androstenedione API manufacturers, exporters, distributors and traders.
click here to find a list of Androstenedione suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Androstenedione DMF (Drug Master File) is a document detailing the whole manufacturing process of Androstenedione active pharmaceutical ingredient (API) in detail. Different forms of Androstenedione DMFs exist exist since differing nations have different regulations, such as Androstenedione USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Androstenedione DMF submitted to regulatory agencies in the US is known as a USDMF. Androstenedione USDMF includes data on Androstenedione's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Androstenedione USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Androstenedione suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Androstenedione as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Androstenedione API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Androstenedione as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Androstenedione and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Androstenedione NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Androstenedione suppliers with NDC on PharmaCompass.
Androstenedione Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Androstenedione GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Androstenedione GMP manufacturer or Androstenedione GMP API supplier for your needs.
A Androstenedione CoA (Certificate of Analysis) is a formal document that attests to Androstenedione's compliance with Androstenedione specifications and serves as a tool for batch-level quality control.
Androstenedione CoA mostly includes findings from lab analyses of a specific batch. For each Androstenedione CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Androstenedione may be tested according to a variety of international standards, such as European Pharmacopoeia (Androstenedione EP), Androstenedione JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Androstenedione USP).

