
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
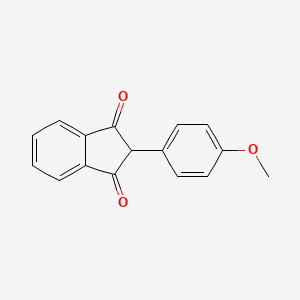

1. 2-(4-methoxyphenyl)-1h-indene-1,3(2h)-dione
2. Miradon
1. 117-37-3
2. Miradon
3. 2-(4-methoxyphenyl)-1h-indene-1,3(2h)-dione
4. Anisin Indandione
5. Unidone
6. 2-p-anisyl-1,3-indandione
7. Andion
8. Anisindionum
9. Anisindiona
10. 2-(4-methoxyphenyl)indene-1,3-dione
11. 1h-indene-1,3(2h)-dione, 2-(4-methoxyphenyl)-
12. 2-(4-methoxyphenyl)indan-1,3-dione
13. 2-(p-methoxyphenyl)indane-1,3-dione
14. 2-para-anisyl-1,3-indandione
15. 2-(p-methoxyphenyl)-1,3-indandione
16. Spe 2792
17. Anisindione (inn)
18. Chembl712
19. Nsc-759629
20. S747t1eraj
21. 1,3-indandione, 2-(p-methoxyphenyl)-
22. 2-(4-methoxy-phenyl)-indan-1,3-dione
23. Mls000554135
24. Chebi:133809
25. Ncgc00094969-01
26. Smr000146452
27. Anisindione [inn]
28. Dsstox_cid_2611
29. Dsstox_rid_76658
30. Dsstox_gsid_22611
31. Anisindionum [inn-latin]
32. Anisindiona [inn-spanish]
33. Cas-117-37-3
34. Miradon (tn)
35. Hsdb 3205
36. Sr-01000642873
37. Einecs 204-186-6
38. Unii-s747t1eraj
39. Brn 1880681
40. Anisindione [inn:ban:nf]
41. 1,3-indanedione, 2-(4-methoxyphenyl)-
42. 2-(4-methoxyphenyl)indane-1,3-dione
43. Spectrum_001355
44. Anisindione [mi]
45. Opera_id_1613
46. Spectrum2_000427
47. Spectrum3_001525
48. Spectrum4_000723
49. Spectrum5_000960
50. Anisindione [hsdb]
51. Anisindione [vandf]
52. Anisindione [mart.]
53. Oprea1_729452
54. Schembl49379
55. Anisindione [who-dd]
56. Bspbio_002910
57. Kbiogr_000986
58. Kbioss_001835
59. 3-08-00-02931 (beilstein Handbook Reference)
60. Mls001201835
61. Divk1c_000336
62. Spectrum1502198
63. 2-[4-(methyloxy)phenyl]-1h-indene-1,3(2h)-dione
64. Spbio_000414
65. Gtpl6960
66. Dtxsid3022611
67. Hms501a18
68. Kbio1_000336
69. Kbio2_001835
70. Kbio2_004403
71. Kbio2_006971
72. Kbio3_002410
73. Anisindione [orange Book]
74. Ninds_000336
75. Hms1921l18
76. Hms2092f04
77. Hms2231b10
78. Hms3369m11
79. Pharmakon1600-01502198
80. Albb-013687
81. Hy-b0924
82. Isopropyl N-acetyl-?-d-glucosamine
83. Tox21_111370
84. Bdbm50280155
85. Ccg-40244
86. Mfcd00176194
87. Nsc759629
88. Stk363125
89. Akos000987728
90. Tox21_111370_1
91. Zinc100015486
92. Db01125
93. Nsc 759629
94. Idi1_000336
95. Ncgc00094969-02
96. Ncgc00094969-03
97. Ncgc00094969-05
98. Bs-52012
99. Sbi-0051739.p002
100. Db-041366
101. Ft-0631668
102. Sw220113-1
103. D07457
104. E77875
105. Ab00052289_10
106. Ab00052289_11
107. J-003611
108. Q3617574
109. Sr-01000642873-1
110. Sr-01000642873-3
111. Sr-01000642873-4
112. 2-(4-methoxyphenyl)-2,3-dihydro-1h-indene-1,3-dione
| Molecular Weight | 252.26 g/mol |
|---|---|
| Molecular Formula | C16H12O3 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 252.078644241 g/mol |
| Monoisotopic Mass | 252.078644241 g/mol |
| Topological Polar Surface Area | 43.4 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 344 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
...Oral anticoagulants are useful in prevention and treatment of variety of thromboembolic disorders. /oral anticoagulants/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1362
...Indications for anticoagulants...myocardial infarction...rheumatic heart Disease...cerebrovascular disease...venous thrombosis and pulmonary embolism, and ...disseminated intravascular coagulation. /oral anticoagulants/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1363
Oral anticoagulants are used to prevent the progression or recurrence of acute deep vein thrombosis or pulmonary embolism following initial course of heparin. They also are effective in preventing venous thromboembolism in patients undergoing orthopedic or gynecological surgery and systemic embolization in patients with acute myocardial infarction, prosthetic heart valves, or chronic atrial fibrillation. /Oral anticoagulants/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1530
Anticoagulants are indicated for prophylaxis and/or treatment of venous (or arterial /Not included in US product labeling/) thrombosis (and its extension) and pulmonary embolism, deep vein thrombosis (DVP) or pulmonary embolism (treatment). Oral anticoagulatns are used during and following initial heparin therapy to decrease the risk of extension, recurrence, or death. /Anticoagulants; Included in US product labeling/
MICROMEDEX Thomson Health Care. USPDI - Drug Information for the Health Care Professional. 22nd ed. Volume 1. MICROMEDEX Thomson Health Care, Greenwood Village, CO. 2002. Content Reviewed and Approved by the U.S. Pharmacopeial Convention, Inc., p. 265
For more Therapeutic Uses (Complete) data for ANISINDIONE (11 total), please visit the HSDB record page.
Serious, sometimes fatal, toxic reactions were reported occasionally in patients receiving phenindione (no longer commercially available in US), and the possibility of these reactions should be considered in patients receiving anisindione. Adverse effects reported with phenindione (no longer commercially available in US) include dermatologic reactions such as such as urticaria and rash (usually erythematous and macular), which sometimes progressed to exfoliative dermatitis; nephrotic reactions, including anuria and albuminuria with massive edema and tubular necrosis; hepatotoxicity manifested by hepatitis and jaundice; and hematologic reactions manifested by eosinophilia, agranulocytosis or leukopenia (resulting from either maturation arrest or granulocytic hypoplasia), leukocytosis, anemia, thrombecytopenia, atypical mononuclear cells, the presence of leukocyte agglutinins, agranulocytosis, aplastic anemia, and red cell aplasia. In addition, hemorrhagic infarction and necrosis of the skin, alopecia, steatorrhea, sore throat and mouth, parlysis of accommodation and blurred vision, diarrhea, nausea, fever, malaise, and headache have been reported in patients receiving the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1417-8
In patients with alkaline urine, the urine may be red-orange during therapy with anisindione and patients should be informed of this possibility.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1418
The only consistently reported adverse nonhemorrhagic effect of anisindione is dermatitis. However, agranulocytosis and hepatitis also have been reported with the use of this drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2002. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2002 (Plus Supplements)., p. 1417
Conversely, anisindione (plasma half-life, 3-5 days).../is/ so long-acting that.../it/ may be Hazardous if hemorrhage occurs...
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 115
For more Drug Warnings (Complete) data for ANISINDIONE (30 total), please visit the HSDB record page.
For the prophylaxis and treatment of venous thrombosis and its extension, the treatment of atrial fibrillation with embolization, the prophylaxis and treatment of pulmonary embolism, and as an adjunct in the treatment of coronary occlusion.
Anisindione is a synthetic anticoagulant and an indanedione derivative. It is prescribed only if you cannot take coumarin-type anticoagulants such as coumadin as anisindione is a powerful drug with serious potential side effects. Anticoagulants decrease the clotting ability of the blood and therefore help to prevent harmful clots from forming in the blood vessels. These medicines are sometimes called blood thinners, although they do not actually thin the blood. They also will not dissolve clots that already have formed, but they may prevent the clots from becoming larger and causing more serious problems.
Absorption
Accumulation does not occur with repeated dosing.
Not Known
...PLASMA HALF-LIFE, 3-5 DAYS...
American Medical Association, AMA Department of Drugs, AMA Drug Evaluations. 3rd ed. Littleton, Massachusetts: PSG Publishing Co., Inc., 1977., p. 115
Like phenindione, to which it is related chemically, anisindione exercises its therapeutic action by reducing the prothrombin activity of the blood. By inhibiting the vitamin K–mediated gamma-carboxylation of precursor proteins, the formation of active procoagulation factors II, VII, IX, and X, as well as the anticoagulant proteins C and S is prevented. Anisindione has no direct thrombolytic effect and does not reverse ischemic tissue damage, although it may limit extension of existing thrombi and prevent secondary thromboembolic complications.
Oral anticoagulants have no direct effect on circulating clotting factors. Instead they block hepatic formation of factors II, VII, IX, and X by competitively inhibiting action of vitamin K. Synthesis of these factors is dependent upon sufficient supply of vitamin.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1356
The oral anticoagulants block the regeneration of reduced vitamin K and thereby induce a state of functional vitamin K deficiency. The mechanism of the inhibition of reductase(s) by the coumarin drugs is not known. There exist reductases that are less sensitive to these drugs but that act only at relatively high concentrations of oxidized vitamin K; this property may explain the observation that administration of sufficient vitamin K can counteract even large doses of oral anticoagulants. /Oral Anticoagulants/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1527
Both 4-hydroxycoumarin derivatives and indandiones (also known as oral anticoagulants) are antagonists of vitamin K. Their use as rodenticides is based on the inhibition of the vitamin K-dependent step in the synthesis of a number of blood coagulation factors. The vitamin K-dependent proteins ...in the coagulation cascade... are the procoagulant factors II (prothrombin), VII (proconvertin), IX (Christmas factor) and X (Stuart-Prower factor), and the coagulation-inhibiting proteins C and S. All these proteins are synthesized in the liver. Before they are released into the circulation the various precursor proteins undergo substantial (intracellular) post-translational modification. Vitamin K functions as a co-enzyme in one of these modifications, namely the carboxylation at well-defined positions of 10-12 glutamate residues into gamma-carboxyglutamate (Gla). The presence of these Gla residues is essential for the procoagulant activity of the various coagulations factors. Vitamin K hydroquinone (KH2) is the active co-enzyme, and its oxidation to vitamin K 2,3-epoxide (KO) provides the energy required for the carboxylation reaction. The epoxide is than recycled in two reduction steps mediated by the enzyme KO reductase... . The latter enzyme is the target enzyme for coumarin anticoagulants. Their blocking of the KO reductase leads to a rapid exhaustion of the supply of KH2, and thus to an effective prevention of the formation of Gla residues. This leads to an accumulation of non-carboxylated coagulation factor precursors in the liver. In some cases these precursors are processed further without being carboxylated, and (depending on the species) may appear in the circulation. At that stage the under-carboxylated proteins are designated as descarboxy coagulation factors. Normal coagulation factors circulate in the form of zymogens, which can only participate in the coagulation cascade after being activated by limited proteolytic degradation. Descarboxy coagulation factors have no procoagulant activity (i.e. they cannot be activated) and neither they can be converted into the active zymogens by vitamin K action. Whereas in anticoagulated humans high levels of circulating descarboxy coagulation factors are detectable, these levels are negligible in warfarin-treated rats and mice. /Anticoagulant rodenticides/
WHO; Environ Health Criteria 175: Anticoagulant Rodenticides p.46 (1995)
ABOUT THIS PAGE
24
PharmaCompass offers a list of Anisindione API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Anisindione manufacturer or Anisindione supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Anisindione manufacturer or Anisindione supplier.
PharmaCompass also assists you with knowing the Anisindione API Price utilized in the formulation of products. Anisindione API Price is not always fixed or binding as the Anisindione Price is obtained through a variety of data sources. The Anisindione Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Anisindione manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Anisindione, including repackagers and relabelers. The FDA regulates Anisindione manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Anisindione API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Anisindione supplier is an individual or a company that provides Anisindione active pharmaceutical ingredient (API) or Anisindione finished formulations upon request. The Anisindione suppliers may include Anisindione API manufacturers, exporters, distributors and traders.
Anisindione Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Anisindione GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Anisindione GMP manufacturer or Anisindione GMP API supplier for your needs.
A Anisindione CoA (Certificate of Analysis) is a formal document that attests to Anisindione's compliance with Anisindione specifications and serves as a tool for batch-level quality control.
Anisindione CoA mostly includes findings from lab analyses of a specific batch. For each Anisindione CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Anisindione may be tested according to a variety of international standards, such as European Pharmacopoeia (Anisindione EP), Anisindione JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Anisindione USP).
