
Synopsis
Synopsis
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
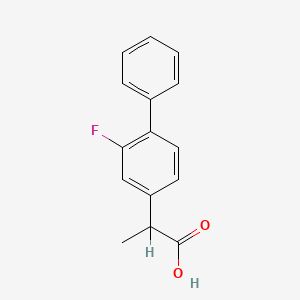

1. 2-fluoro-alpha-methyl-(1,1'-biphenyl)-4-acetic Acid
2. Ansaid
3. Apo Flurbiprofen
4. Apo-flurbiprofen
5. Bts 18322
6. Bts-18322
7. Bts18322
8. Cebutid
9. Dobrofen
10. E 7869
11. E-7869
12. E7869
13. Flubiprofen
14. Flugalin
15. Flurbiprofen Sodium
16. Fluriproben
17. Froben
18. Froben Sr
19. Neo Artrol
20. Novo Flurprofen
21. Novo-flurprofen
22. Nu Flurbiprofen
23. Nu-flurbiprofen
24. Ocufen
25. Ocuflur
26. Ratio Flurbiprofen
27. Ratio-flurbiprofen
28. Strefen
1. 5104-49-4
2. Ansaid
3. Froben
4. Antadys
5. 2-(2-fluorobiphenyl-4-yl)propanoic Acid
6. Cebutid
7. Flurofen
8. 3-fluoro-4-phenylhydratropic Acid
9. 2-(3-fluoro-4-phenylphenyl)propanoic Acid
10. Ocufen
11. Flurbiprofene [inn-french]
12. Flurbiprofenum [inn-latin]
13. Flurbiprofeno [inn-spanish]
14. Bts 18322
15. Bts-18322
16. Fp 70
17. U-27182
18. 2-fluoro-alpha-methyl-(1,1'-biphenyl)-4-acetic Acid
19. (+-)-2-fluoro-alpha-methyl-4-biphenylacetic Acid
20. 2-(2-fluorobiphenyl-4-yl)propionic Acid
21. Mfcd00079303
22. Stayban
23. Zepolas
24. Adfeed
25. U 27182
26. 2-(2-fluoro-[1,1'-biphenyl]-4-yl)propanoic Acid
27. 51543-38-5
28. Chembl563
29. Bts 18,322
30. Nsc-757037
31. 5gro578klp
32. 2-(2-fluoro-4-biphenylyl)propionic Acid
33. (r)-flurbiprofen;mpc7869
34. Mls000040873
35. Chebi:5130
36. Flubiprofen
37. Flugalin
38. Einecs 257-262-6
39. U-27,182
40. (1)-2-fluoro-alpha-methyl(1,1'-biphenyl)-4-acetic Acid
41. Flurbiprofene
42. Flurbiprofeno
43. Flurbiprofenum
44. 2-fluoro-alpha-methyl-4-biphenylacetic Acid
45. Ocuflur
46. Smr000042823
47. Dsstox_cid_17231
48. Dsstox_rid_79310
49. Dsstox_gsid_37231
50. C15h13fo2
51. Anmetarin
52. Yakuban
53. Dl-flurbiprofen
54. Flurbiprofen (ansaid)
55. Ansaid (tn)
56. Ccris 3708
57. Sr-01000003043
58. Flp
59. Einecs 225-827-6
60. (+-)-2-(2-fluoro-4-biphenylyl)propionic Acid
61. Unii-5gro578klp
62. (+/-)-2-fluoro-alpha-methyl-4-biphenylacetic Acid
63. Mpc7869
64. U 27,182
65. Flurbiprofen O
66. Mks-11
67. Rac-flurbiprofen
68. Flurbiprofen,(s)
69. Ncgc00016654-01
70. (+-)flurbiprofen
71. Cas-5104-49-4
72. Ocufen (salt/mix)
73. [1,1'-biphenyl]-4-acetic Acid, 2-fluoro-?-methyl-
74. Flurbiprofen [usan:usp:inn:ban:jan]
75. Spectrum_001096
76. Opera_id_777
77. Prestwick0_000917
78. Prestwick1_000917
79. Prestwick2_000917
80. Prestwick3_000917
81. Spectrum2_001025
82. Spectrum3_000435
83. Spectrum4_000558
84. Spectrum5_000720
85. Flurbiprofen [mi]
86. 2-(3-fluoro-4-phenyl-phenyl)propanoic Acid
87. F0371
88. (.+/-.)-flurbiprofen
89. Flurbiprofen [inn]
90. Flurbiprofen [jan]
91. 4-biphenylacetic Acid, 2-fluoro-alpha-methyl-
92. Flurbiprofen [usan]
93. Schembl2248
94. Flurbiprofen [vandf]
95. 4-biphenylacetic Acid, 2-fluoro-.alpha.-methyl-
96. Bspbio_000794
97. Bspbio_002050
98. Flurbiprofen [mart.]
99. Kbiogr_001255
100. Kbioss_001576
101. 2-(2-fluoro[1,1'-biphenyl]-4-yl)propanoic Acid
102. Mls000028441
103. Mls000758198
104. Mls001201729
105. Mls001401361
106. Mls006011431
107. Mls006011931
108. Divk1c_000804
109. Flurbiprofen [usp-rs]
110. Flurbiprofen [who-dd]
111. Spectrum1500308
112. 2-(2-fluoro-[1,1'-biphenyl-4-yl])propanoic Acid
113. Spbio_001209
114. Spbio_002983
115. Bpbio1_000874
116. Gtpl4194
117. Dtxsid0037231
118. Schembl10029029
119. Flurbiprofen (jp17/usp/inn)
120. Hms502i06
121. Kbio1_000804
122. Kbio2_001576
123. Kbio2_004144
124. Kbio2_006712
125. Kbio3_001270
126. Sytbzmrglbwntm-uhfffaoysa-
127. [1,1'-biphenyl]-4-acetic Acid, 2-fluoro-.alpha.-methyl-
128. Ninds_000804
129. Flurbiprofen [orange Book]
130. Hms1570h16
131. Hms1920o20
132. Hms2051a05
133. Hms2090i06
134. Hms2091f21
135. Hms2097h16
136. Hms2232i08
137. Hms3259i12
138. Hms3268e10
139. Hms3370n14
140. Hms3371i05
141. Hms3393a05
142. Hms3414n17
143. Hms3649k11
144. Hms3655g19
145. Hms3678n15
146. Hms3714h16
147. Pharmakon1600-01500308
148. Flurbiprofen [ep Monograph]
149. (1,1'-biphenyl)-4-acetic Acid, 2-fluoro-alpha-methyl-, (+-)-
150. Bcp09086
151. Bcp13426
152. Flurbiprofen [usp Monograph]
153. Tox21_110547
154. Tox21_302353
155. Bbl010980
156. Bdbm50074922
157. Ccg-40243
158. Flurbiprofen - Cas 5104-49-4
159. Nsc685701
160. Nsc755404
161. Nsc757037
162. Stk802101
163. Flurbiprofen, Cyclooxygenase Inhibitor
164. Akos004119934
165. Akos016340701
166. Ac-8106
167. Ccg-100759
168. Db00712
169. Ks-5035
170. Nc00009
171. Nc00563
172. Nsc 757037
173. Nsc-685701
174. Nsc-755404
175. 2(2-fluoro-4-biphenylyl)propionic Acid
176. Idi1_000804
177. Ncgc00018157-03
178. Ncgc00018157-04
179. Ncgc00018157-05
180. Ncgc00018157-08
181. Ncgc00018157-11
182. Ncgc00018157-13
183. Ncgc00025287-03
184. Ncgc00025287-04
185. Ncgc00255457-01
186. Hy-10582
187. Nci60_030812
188. 2 -(2-fluoro-4-biphenylyl)propionic Acid
189. 2-(2-fluoro-4-biphenylyl)-propionic Acid
190. 2-(2-fluoro-biphenyl-4-yl)propionic Acid
191. Sbi-0051387.p003
192. Db-051888
193. Flurbiprofen 100 Microg/ml In Acetonitrile
194. 2-(2-fluoro-biphenyl-4-yl)-propionic Acid
195. Ab00051999
196. Ft-0603668
197. Ft-0660285
198. Ft-0668760
199. Ft-0771103
200. Alpha-methyl-2-fluoro-4-biphenylylacetic Acid
201. (rs)-2-(2-fluorobiphenyl-4-yl)propionic Acid
202. 2-fluoro-.alpha.-methyl-4-biphenylacetic Acid
203. Bim-0051387.0001
204. C07013
205. D00330
206. Ab00051999-17
207. Ab00051999_18
208. 104f494
209. 2-(2-fluoro-1,1'-biphenyl-4-yl)propanoic Acid
210. 2-fluoro--methyl-(1,1'-biphenyl)-4-acetic Acid
211. Ao-295/42284050
212. Q419890
213. (.+/-.)-2-(2-fluoro-4-biphenylyl)propionic Acid
214. (r/s)-2-fluoro-alpha-methyl-4-biphenylacetic Acid
215. 2-fluoro-?-methyl-[1,1'-biphenyl]-4-acetic Acid
216. Flurbiprofen, Antibiotic For Culture Media Use Only
217. Q-201129
218. Sr-01000003043-2
219. Sr-01000003043-5
220. Sr-01000003043-6
221. (s)-(+)-2-fluoro-alpha-methyl-4-biphenylaceticacid
222. 2-(2-fluoro-[1,1''-biphenyl-4-yl])propanoic Acid
223. 2-(2-fluoro[1,1'-biphenyl]-4-yl)propanoic Acid #
224. Brd-a86044036-001-05-9
225. Sr-01000003043-14
226. (+/-)-2-(2-fluoro-4-biphenylyl)propionic Acid
227. (.+/-.)-2-fluoro-.alpha.-methyl-4-biphenylacetic Acid
228. 2-fluoro-.alpha.-methyl(1,1'-biphenyl)-4-acetic Acid
229. 2-fluoro-alpha-methyl-(1,1''-biphenyl)-4-acetic Acid
230. 2-fluoro-alpha-methyl-[1,1'-biphenyl]-4-a Cetic Acid
231. (+/-)-2-fluoro-.alpha.-methyl-4-biphenylacetic Acid
232. Flurbiprofen, European Pharmacopoeia (ep) Reference Standard
233. (1,1'-biphenyl)-4-acetic Acid, 2-fluoro-alpha-methyl-, (+/-)-
234. Flurbiprofen, United States Pharmacopeia (usp) Reference Standard
235. [1,1'-biphenyl]-4-acetic Acid, 2-fluoro-.alpha.-methyl-, (.+/-.)-
236. (1,1'-biphenyl)-4-acetic Acid, 2-fluoro-.alpha.-methyl-, (+/-)-
237. Flurbiprofen, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 244.26 g/mol |
|---|---|
| Molecular Formula | C15H13FO2 |
| XLogP3 | 4.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 244.08995782 g/mol |
| Monoisotopic Mass | 244.08995782 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 286 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Ansaid |
| PubMed Health | Flurbiprofen |
| Drug Classes | Analgesic, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, NSAID, Ophthalmologic Agent, Propionic Acid |
| Drug Label | Flurbiprofen tablet, USP contain flurbiprofen, which is a member of the phenylalkanoic acid derivative group of nonsteroidal anti-inflammatory drugs. Flurbiprofen tablet, USP are white, oval, film-coated tablets for oral administration. Flurbiprofen... |
| Active Ingredient | Flurbiprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 50mg |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 2 of 6 | |
|---|---|
| Drug Name | Flurbiprofen |
| PubMed Health | Flurbiprofen |
| Drug Classes | Analgesic, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, NSAID, Ophthalmologic Agent, Propionic Acid |
| Drug Label | OCUFEN (flurbiprofen sodium ophthalmic solution, USP) 0.03% is a sterile topical nonsteroidal anti-inflammatory product for ophthalmic use. Chemical Name:Sodium ()-2-(2-fluoro-4-biphenylyl) propionate dihydrate. Structural Formula:C15H12FNaO22... |
| Active Ingredient | Flurbiprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 50mg |
| Market Status | Prescription |
| Company | Teva; Sun Pharm Inds; Mylan |
| 3 of 6 | |
|---|---|
| Drug Name | Ocufen |
| PubMed Health | Flurbiprofen |
| Drug Classes | Analgesic, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, NSAID, Ophthalmologic Agent, Propionic Acid |
| Active Ingredient | Flurbiprofen sodium |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.03% |
| Market Status | Prescription |
| Company | Allergan |
| 4 of 6 | |
|---|---|
| Drug Name | Ansaid |
| PubMed Health | Flurbiprofen |
| Drug Classes | Analgesic, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, NSAID, Ophthalmologic Agent, Propionic Acid |
| Drug Label | Flurbiprofen tablet, USP contain flurbiprofen, which is a member of the phenylalkanoic acid derivative group of nonsteroidal anti-inflammatory drugs. Flurbiprofen tablet, USP are white, oval, film-coated tablets for oral administration. Flurbiprofen... |
| Active Ingredient | Flurbiprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 50mg |
| Market Status | Prescription |
| Company | Pharmacia And Upjohn |
| 5 of 6 | |
|---|---|
| Drug Name | Flurbiprofen |
| PubMed Health | Flurbiprofen |
| Drug Classes | Analgesic, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, NSAID, Ophthalmologic Agent, Propionic Acid |
| Drug Label | OCUFEN (flurbiprofen sodium ophthalmic solution, USP) 0.03% is a sterile topical nonsteroidal anti-inflammatory product for ophthalmic use. Chemical Name:Sodium ()-2-(2-fluoro-4-biphenylyl) propionate dihydrate. Structural Formula:C15H12FNaO22... |
| Active Ingredient | Flurbiprofen |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 100mg; 50mg |
| Market Status | Prescription |
| Company | Teva; Sun Pharm Inds; Mylan |
| 6 of 6 | |
|---|---|
| Drug Name | Ocufen |
| PubMed Health | Flurbiprofen |
| Drug Classes | Analgesic, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, NSAID, Ophthalmologic Agent, Propionic Acid |
| Active Ingredient | Flurbiprofen sodium |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 0.03% |
| Market Status | Prescription |
| Company | Allergan |
Flurbiprofen tablets are indicated for the acute or long-term symptomatic treatment of rheumatoid arthritis, osteorarthritis and anklosing spondylitis. It may also be used to treat pain associated with dysmenorrhea and mild to moderate pain accompanied by inflammation (e.g. bursitis, tendonitis, soft tissue trauma). Topical ophthalmic formulations may be used pre-operatively to prevent intraoperative miosis.
FDA Label
Flurbiprofen, a nonsteroidal anti-inflammatory agent (NSAIA) of the propionic acid class, is structually and pharmacologically related to fenoprofen, ibuprofen, and ketoprofen, and has similar pharmacological actions to other prototypica NSAIAs. Flurbiprofen exhibits antiinflammatory, analgesic, and antipyretic activities. The commercially available flurbiprofen is a racemic mixture of (+)S- and (-) R-enantiomers. The S-enantiomer appears to possess most of the anti-inflammatory, while both enantiomers may possess analgesic activity.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
Analgesics
Compounds capable of relieving pain without the loss of CONSCIOUSNESS. (See all compounds classified as Analgesics.)
R02AX01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AE - Propionic acid derivatives
M01AE09 - Flurbiprofen
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AA - Antiinflammatory preparations, non-steroids for topical use
M02AA19 - Flurbiprofen
R - Respiratory system
R02 - Throat preparations
R02A - Throat preparations
R02AX - Other throat preparations
R02AX01 - Flurbiprofen
S - Sensory organs
S01 - Ophthalmologicals
S01B - Antiinflammatory agents
S01BC - Antiinflammatory agents, non-steroids
S01BC04 - Flurbiprofen
Absorption
Fluribiprofen is rapidly and almost completely absorbed following oral administration. Peak plasma concentrations are reached 0.5 - 4 hours after oral administration.
Route of Elimination
Flurbiprofen is poorly excreted into human milk. Following dosing with flurbiprofen, less than 3% of flurbiprofen is excreted unchanged in the urine, with about 70% of the dose eliminated in the urine as parent drug and metabolites. Renal elimination is a significant pathway of elimination of flurbiprofen metabolites.
Volume of Distribution
14 L [Normal Healthy Adults]
12 L [Geriatric Arthritis Patients]
10 L [End Stage Renal Disease Patients]
14 L [Alcoholic Cirrhosis Patients]
0.12 L/kg
Hepatic. Cytochrome P450 2C9 plays an important role in the metabolism of flurbiprofen to its major metabolite, 4’-hydroxy-flurbiprofen. The 4’-hydroxy-flurbiprofen metabolite showed little anti-inflammatory activity in animal models of inflammation.
R-flurbiprofen, 4.7 hours; S-flurbiprofen, 5.7 hours
Similar to other NSAIAs, the anti-inflammatory effect of flurbiprofen occurs via reversible inhibition of cyclooxygenase (COX), the enzyme responsible for the conversion of arachidonic acid to prostaglandin G2 (PGG2) and PGG2 to prostaglandin H2 (PGH2) in the prostaglandin synthesis pathway. This effectively decreases the concentration of prostaglandins involved in inflammation, pain, swelling and fever. Flurbiprofen is a non-selective COX inhibitor and inhibits the activity of both COX-1 and -2. It is also one of the most potent NSAIAs in terms of prostaglandin inhibitory activity.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
38
PharmaCompass offers a list of Flurbiprofen API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Flurbiprofen manufacturer or Flurbiprofen supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Flurbiprofen manufacturer or Flurbiprofen supplier.
PharmaCompass also assists you with knowing the Flurbiprofen API Price utilized in the formulation of products. Flurbiprofen API Price is not always fixed or binding as the Flurbiprofen Price is obtained through a variety of data sources. The Flurbiprofen Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Anside manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Anside, including repackagers and relabelers. The FDA regulates Anside manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Anside API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Anside manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Anside supplier is an individual or a company that provides Anside active pharmaceutical ingredient (API) or Anside finished formulations upon request. The Anside suppliers may include Anside API manufacturers, exporters, distributors and traders.
click here to find a list of Anside suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Anside DMF (Drug Master File) is a document detailing the whole manufacturing process of Anside active pharmaceutical ingredient (API) in detail. Different forms of Anside DMFs exist exist since differing nations have different regulations, such as Anside USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Anside DMF submitted to regulatory agencies in the US is known as a USDMF. Anside USDMF includes data on Anside's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Anside USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Anside suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Anside Drug Master File in Japan (Anside JDMF) empowers Anside API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Anside JDMF during the approval evaluation for pharmaceutical products. At the time of Anside JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Anside suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Anside Drug Master File in Korea (Anside KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Anside. The MFDS reviews the Anside KDMF as part of the drug registration process and uses the information provided in the Anside KDMF to evaluate the safety and efficacy of the drug.
After submitting a Anside KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Anside API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Anside suppliers with KDMF on PharmaCompass.
A Anside CEP of the European Pharmacopoeia monograph is often referred to as a Anside Certificate of Suitability (COS). The purpose of a Anside CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Anside EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Anside to their clients by showing that a Anside CEP has been issued for it. The manufacturer submits a Anside CEP (COS) as part of the market authorization procedure, and it takes on the role of a Anside CEP holder for the record. Additionally, the data presented in the Anside CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Anside DMF.
A Anside CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Anside CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Anside suppliers with CEP (COS) on PharmaCompass.
A Anside written confirmation (Anside WC) is an official document issued by a regulatory agency to a Anside manufacturer, verifying that the manufacturing facility of a Anside active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Anside APIs or Anside finished pharmaceutical products to another nation, regulatory agencies frequently require a Anside WC (written confirmation) as part of the regulatory process.
click here to find a list of Anside suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Anside as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Anside API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Anside as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Anside and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Anside NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Anside suppliers with NDC on PharmaCompass.
Anside Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Anside GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Anside GMP manufacturer or Anside GMP API supplier for your needs.
A Anside CoA (Certificate of Analysis) is a formal document that attests to Anside's compliance with Anside specifications and serves as a tool for batch-level quality control.
Anside CoA mostly includes findings from lab analyses of a specific batch. For each Anside CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Anside may be tested according to a variety of international standards, such as European Pharmacopoeia (Anside EP), Anside JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Anside USP).
