
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
NDC API
0
VMF
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
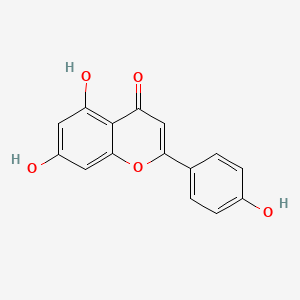

1. 520-36-5
2. 5,7-dihydroxy-2-(4-hydroxyphenyl)-4h-chromen-4-one
3. Chamomile
4. Versulin
5. 4',5,7-trihydroxyflavone
6. Apigenol
7. Apigenine
8. Spigenin
9. C.i. Natural Yellow 1
10. 5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one
11. 5,7,4'-trihydroxyflavone
12. 5,7-dihydroxy-2-(4-hydroxyphenyl)-4h-1-benzopyran-4-one
13. Pelargidenon 1449
14. 5,7-dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone
15. Nsc 83244
16. 2-(p-hydroxyphenyl)-5,7-dihydroxychromone
17. 4h-1-benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxyphenyl)-
18. Uccf 031
19. Flavone, 4',5,7-trihydroxy-
20. Chebi:18388
21. Chembl28
22. Mfcd00006831
23. Nsc-83244
24. 8002-66-2
25. 7v515pi7f6
26. Nsc83244
27. Cas-520-36-5
28. Dsstox_cid_2391
29. Dsstox_rid_76568
30. Dsstox_gsid_22391
31. 4',5,7-trihydroxyflavone;apigenol;c.i. Natural Yellow 1
32. Smr000326850
33. Ccris 3789
34. 4′,5,7-trihydroxyflavone
35. Sr-01000075663
36. Einecs 208-292-3
37. Brn 0262620
38. Pelargidenone
39. Matricaria Oil
40. Unii-7v515pi7f6
41. Chamomile Powder
42. Hsdb 7573
43. 4der
44. 4dgm
45. 4hkk
46. Naringenin, 18
47. Prestwick_719
48. Apigenin, 13
49. Apegenin
50. Tocris-1227
51. 3cf9
52. St056301
53. Apigenin [hsdb]
54. Apigenin [inci]
55. 4',7-trihydroxyflavone
56. Apigenin [mi]
57. Biomolki_000078
58. Prestwick0_000414
59. Prestwick1_000414
60. Prestwick2_000414
61. Prestwick3_000414
62. Spectrum2_000428
63. Spectrum3_001882
64. Spectrum4_001999
65. Lopac-a-3145
66. Apigenin [usp-rs]
67. Apigenin [who-dd]
68. Biomolki2_000082
69. 4,5, 7-trihydroxyflavone
70. Pelargidenon-1449
71. Ci Natural Yellow 1
72. Lopac0_000065
73. Oprea1_622293
74. Schembl19428
75. 4',5,7-trihydroxy-flavone
76. Apigenin, Analytical Standard
77. Bspbio_000368
78. Bspbio_003384
79. Kbiogr_002565
80. Spectrum200846
81. 5-18-04-00574 (beilstein Handbook Reference)
82. Mls000697626
83. Mls000859991
84. Mls001074874
85. Mls006011839
86. Bidd:er0135
87. Divk1c_000798
88. Schembl222227
89. Spbio_000416
90. Spbio_002307
91. Ghl.pd_mitscher_leg0.1194
92. Bdbm7458
93. Bpbio1_000406
94. Gtpl4136
95. Megxp0_000176
96. Uccf-031
97. Dtxsid6022391
98. Acon1_002450
99. Cid_5280443
100. Hms502h20
101. Kbio1_000798
102. Kbio3_002887
103. Ninds_000798
104. Bio1_000376
105. Bio1_000865
106. Bio1_001354
107. Hms1569c10
108. Hms1922p22
109. Hms2096c10
110. Hms2230d17
111. Hms3260m11
112. Hms3267d21
113. Hms3373b18
114. Hms3412a08
115. Hms3561p09
116. Hms3655d18
117. Hms3676a08
118. Hms3866d03
119. Apigenin, >=95.0% (hplc)
120. 4',5,7-trihydroxyflavone, 97%
121. Bcp28288
122. Hy-n1201
123. Zinc3871576
124. Tox21_201542
125. Tox21_302884
126. Tox21_500065
127. Apigenin; 4',5,7-trihydroxyflavone
128. Bbl010499
129. Ccg-40061
130. Hsci1_000221
131. Lmpk12110005
132. Nsc815095
133. S2262
134. Stk801630
135. Zb1873
136. Akos002140699
137. Ac-8011
138. Cs-5432
139. Db07352
140. Lp00065
141. Nd-9076
142. Nsc-815095
143. Sdccgmls-0066379.p001
144. Sdccgsbi-0050053.p003
145. Idi1_000798
146. Smp2_000338
147. Apigenin, >=97% (tlc), From Citrus
148. Ncgc00015049-01
149. Ncgc00015049-02
150. Ncgc00015049-03
151. Ncgc00015049-04
152. Ncgc00015049-05
153. Ncgc00015049-06
154. Ncgc00015049-07
155. Ncgc00015049-08
156. Ncgc00015049-09
157. Ncgc00015049-10
158. Ncgc00015049-11
159. Ncgc00015049-12
160. Ncgc00015049-13
161. Ncgc00015049-14
162. Ncgc00015049-15
163. Ncgc00015049-16
164. Ncgc00015049-18
165. Ncgc00015049-28
166. Ncgc00025057-01
167. Ncgc00025057-02
168. Ncgc00025057-03
169. Ncgc00025057-04
170. Ncgc00025057-05
171. Ncgc00025057-06
172. Ncgc00025057-07
173. Ncgc00025057-08
174. Ncgc00025057-09
175. Ncgc00169835-01
176. Ncgc00169835-02
177. Ncgc00169835-03
178. Ncgc00256419-01
179. Ncgc00259092-01
180. Ncgc00260750-01
181. Ly080400
182. Nci60_041830
183. Sy005957
184. Ts-00897
185. Ly 080400
186. Ly-080400
187. Eu-0100065
188. Ft-0622445
189. Ft-0623582
190. Ft-0662251
191. N1828
192. Sw196866-2
193. 20a365
194. A 3145
195. C01477
196. K00045
197. O11338
198. Apigenin 100 Microg/ml In Acetonitrile:methanol
199. Apigenin, >=97% (tlc), From Parsley, Powder
200. Biochem Biophys Res Comm 212: 767 (1997)
201. 5,7-dihydroxy-2-(4-hydroxyphenyl)-chromen-4-one
202. A828903
203. Apigenin (constituent Of Chamomile) [dsc]
204. Apigenin, Primary Pharmaceutical Reference Standard
205. Q424567
206. 4 Inverted Exclamation Mark ,5,7-trihydroxyflavone
207. Q-100586
208. Q-200822
209. Sr-01000075663-1
210. Sr-01000075663-3
211. Sr-01000075663-7
212. Sr-01000075663-8
213. Brd-k01493881-001-10-4
214. Brd-k01493881-001-17-9
215. 5,7-dihydroxy-2-(4-hydroxyphenyl)-4h-chromen-4-one #
216. 4h-1-benzopyran-4-one,7-dihydroxy-2-(4-hydroxyphenyl)-
217. D50a2d8a-6d8b-4708-b21e-2de9580d033f
218. Apigenin, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 270.24 g/mol |
|---|---|
| Molecular Formula | C15H10O5 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 1 |
| Exact Mass | 270.05282342 g/mol |
| Monoisotopic Mass | 270.05282342 g/mol |
| Topological Polar Surface Area | 87 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 411 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ ... This report ... shows ... that apigenin markedly induces the expression of death receptor 5 (DR5) and synergistically acts with exogenous soluble recombinant human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to induce apoptosis in malignant tumor cells. TRAIL is a promising candidate for cancer therapeutics due to its ability to selectively induce apoptosis in cancer cells. The combined use of apigenin and TRAIL at suboptimal concentrations induces Bcl-2-interacting domain cleavage and the activation of caspases-8, -10, -9, and -3. Furthermore, human recombinant DR5/Fc chimera protein and caspase inhibitors dramatically inhibit apoptosis induced by the combination of apigenin and TRAIL. On the other hand, apigenin-mediated induction of DR5 expression is not observed in normal human peripheral blood mononuclear cells. Moreover, apigenin does not sensitize normal human peripheral blood mononuclear cells to TRAIL-induced apoptosis. These results suggest that this combined treatment with apigenin and TRAIL might be promising as a new therapy against malignant tumors.
PMID:16648565 Horinaka M et al; Mol Cancer Ther 5(4):945-51 (2006)
/EXPL THER/ ... The protective role of apigenin was examined against the oxidative stress caused by N-nitrosodiethylamine (NDEA) and phenobarbital (PB) in Wistar albino rats. Oxidative stress was measured in terms of lipid peroxidation (LPO) and protein carbonyl formation. Oxidative stress-induced DNA damage was measured by single cell gel electrophoresis (comet assay). Apigenin exhibited its antioxidant defense against NDEA-induced oxidative stress ... Minimal levels of LPO and DNA damage in apigenin-treated hepatoma bearing animals /were observed/.
PMID:15924350 Jeyabal PV et al; Mol Carcinog 44(1):11-20 (2005)
Four hours after administration of a flavonoid glycoside extract (corresponding to 0.942 mg aglycones) by gavage, the aglycone of apigenin was observed in the lumen and the wall of the stomach, in the lumen of the small intestine and in the lumen and wall of the cecum in Wistar rats. The evidence of glycosides in the stomach wall suggested that the absorption of flavonoids did not require the presence of their aglycones. Under the study conditions, no renal excretion of apigenin was detected ...
Pforte H et al; Nahrung 43(3): 205-208 (1999). As cited in SUMMARY OF DATA FOR CHEMICAL SELECTION: Apigenin 19p. (2000) prepared for NCI. Available from, as of March 20, 2008: https://ntp-server.niehs.nih.gov/
Apigenin appears to be absorbable by humans after intake of parsley (Petroselinum crispum). In a randomized crossover study with two one-week intervention periods in succession, fourteen volunteers consumed a diet that included 20 g parsley. The urinary excretion of apigenin was significantly higher (P < 0.05) during the intervention with parsley (20.7 to 5727.3 g/24 hr) than during the basic diet (0 to 1571.7 g/24 hr). The half-life for apigenin was calculated to be on the order of 12 hr. Significant individual variation in the bioavailability and excretion of apigenin was observed ...
Nielsen SE et al; Br J Nutr 81: 447-455 (1999). As cited in SUMMARY OF DATA FOR CHEMICAL SELECTION: Apigenin 19p. (2000) prepared for NCI. Available from, as of March 20, 2008: https://ntp-server.niehs.nih.gov/
... Eleven healthy subjects (5 women, 6 men) in the age range of 23 to 41 years and with an average body mass index of 23.9 + or - 4.1 kg/sq m took part in this study. After an apigenin- and luteolin-free diet, a single oral bolus of 2 g blanched parsley (corresponding to 65.8 + or - 15.5 umol apigenin) per kilogram body weight was consumed. Blood samples were taken at 0, 4, 6, 7, 8, 9, 10, 11 and 28 hr after parsley consumption and 24-hour urine samples were collected ... On average, a maximum apigenin plasma concentration of 127 + or - 81 nmol/L was reached after 7.2 + or - 1.3 hr with a high range of variation between subjects. For all participants, plasma apigenin concentration rose after bolus ingestion and fell within 28 hr under the detection limit (2.3 nmol/L). The average apigenin content in 24-hour urine was 144 + or - 110 nmol/24 hr corresponding to 0.22 + or - 0.16% of the ingested dose. The flavone could be detected in red blood cells without showing dose-response characteristics.
PMID:16407641 Meyer H et al; Ann Nutr Metab 50(3):167-72 (2006)
... The present paper shows the study of the absorption and excretion of luteolin and apigenin in rats after a single oral dose of Chrysanthemum morifolium extract (CME) (200 mg/kg). The levels of luteolin and apigenin in plasma, urine, feces, and bile were measured by HPLC after deconjugation with hydrochloric acid or beta-glucuronidase/sulfatase. The results showed that the plasma concentrations of luteolin and apigenin reached the highest peak level at 1.1 and 3.9 hr after dosing, respectively. The area under the concentration-time curves (AUC) for luteolin and apigenin were 23.03 and 237.6 ug h/mL, respectively. The total recovery of the dose was 37.9% (6.6% in urine; 31.3% in feces) for luteolin and 45.2% (16.6% in urine; 28.6% in feces) for apigenin. The cumulative luteolin and apigenin excreted in the bile was 2.05% and 6.34% of the dose, respectively. All of the results suggest apigenin may be absorbed more efficiently than luteolin in CME in rats, and both luteolin and apigenin have a slow elimination phase, with a quick absorption, so a possible accumulation of the two flavonoids in the body can be hypothesized.
PMID:17227053 Chen T et al; J Agric Food Chem 55(2):273-7 (2007)
After a single oral administration of radiolabeled apigenin /to rats/, 51.0% of radioactivity was recovered in urine, 12.0% in feces, 1.2% in the blood, 0.4% in the kidneys, 9.4% in the intestine, 1.2% in the liver, and 24.8% in the rest of the body within 10 days. Sex differences appear with regard to the nature of compounds eliminated via the urinary route: immature male and female rats, like mature female rats, excreted a higher percentage of the mono-glucuronoconjugate of apigenin than the mono-sulfoconjugate of apigenin (10.0 to 31.6% versus 2.0 to 3.6%, respectively). Mature male rats excreted the same compounds in an inverse ratio (4.9% and 13.9%, respectively). Radioactivity appeared in the blood only 24 hr after oral administration. Blood kinetics showed a high elimination half-time (91.8 hr), a distribution volume of 259 mL, and a plasmatic clearance of 1.95 mL/hr. All of the parameters calculated from these experiments suggested a slow metabolism of apigenin, with a slow absorption and a slow elimination phase. Thus, a possible accumulation of this flavonoid in the body can be hypothesized.
PMID:15466493 Gradolatto A et al; Drug Metab Dispos 33(1):49-54 (2005)
Ether extracts of the urine of male Wistar rats administered apigenin (200 mg) orally contained the phenolic acid metabolites phydroxyphenylpropionic acid, p-hydroxycinnamic acid, and p-hydroxybenzoic acid. Unreacted apigenin, partially characterized apigenin glucuronides, and ethereal sulfates were also identified. With the exception of p-hydroxybenzoic acid and the apigenin conjugates, all of the metabolites detected in the urine after oral administration were also formed in vitro by rat intestinal microorganisms under anaerobic conditions ... In contrast, these metabolites were not detected in SENCAR mice treated topically with apigenin. Furthermore, no evidence of metabolites were observed from the HPLC profiles of epidermal extracts from apigenin-treated mice ...
Griffiths LA, Smith GE; Biochem J 128: 901-911 (1972). As cited in SUMMARY OF DATA FOR CHEMICAL SELECTION: Apigenin 19p. (2000) prepared for NCI. Available from, as of March 20, 2008: https://ntp-server.niehs.nih.gov/
The main in vitro metabolite of apigenin in rat liver Aroclor 1254-induced microsomes has been identified tentatively as the corresponding 3'-hydroxylated compound, luteolin. Apigenin itself is the 3'-hydroxylated metabolite of chrysin ...
Nielsen SE et al; Xenobiotica 28(4): 389-401 (1998). As cited in SUMMARY OF DATA FOR CHEMICAL SELECTION: Apigenin 19p. (2000) prepared for NCI. Available from, as of March 20, 2008: https://ntp-server.niehs.nih.gov/
Apigenin has known human metabolites that include (2S,3S,4S,5R)-3,4,5-Trihydroxy-6-[5-hydroxy-2-(4-hydroxyphenyl)-4-oxochromen-7-yl]oxyoxane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
... The half-life for apigenin was calculated to be on the order of 12 hr ...
Nielsen SE et al; Br J Nutr 81: 447-455 (1999). As cited in SUMMARY OF DATA FOR CHEMICAL SELECTION: Apigenin 19p. (2000) prepared for NCI. Available from, as of March 20, 2008: https://ntp-server.niehs.nih.gov/
The dietary flavonoid apigenin (Api) has been demonstrated to exert multiple beneficial effects upon the vascular endothelium. The aim of this study was to examine whether Ca(2+)-activated K(+) channels (K(Ca)) are involved in endothelial nitric oxide (NO) production and antiangiogenic effects ... Endothelial NO generation was monitored using a cyclic guanosine monophosphate radioimmunoassay. K(Ca) activity and changes of the intracellular Ca(2+) concentration [Ca(2+)](i) were analyzed using the fluorescent dyes bis-barbituric acid oxonol, potassium-binding benzofuran isophthalate, and fluo-3. The endothelial angiogenic parameters measured were cell proliferation, [(3)H]-thymidine incorporation, and cell migration (scratch assay). Akt phosphorylation was examined using immunohistochemistry ... Api caused a concentration-dependent increase in cyclic guanosine monophosphate levels, with a maximum effect at a concentration of 1 uM. Api-induced hyperpolarization was blocked by the small and large conductance K(Ca) inhibitors apamin and iberiotoxin, respectively. Furthermore, apamin and iberiotoxin blocked the late, long-lasting plateau phase of the Api-induced biphasic increase of [Ca(2+)](i). Inhibition of Ca(2+) signaling and the K(Ca) blockade both blocked NO production. Prevention of all three (NO, Ca(2+), and K(Ca) signaling) reversed the antiangiogenic effects of Api under both basal and basic fibroblast growth factor-induced culture conditions. Basic fibroblast growth factor-induced Akt phosphorylation was also reduced by Api ... Based on ... /the/ experimental results ... /the authors/ propose the following signaling cascade for the effects of Api on endothelial cell signaling. Api activates small and large conductance K(Ca), leading to a hyperpolarization that is followed by a Ca(2+) influx. The increase of [Ca(2+)](i) is responsible for an increased NO production that mediates the antiangiogenic effects of Api via Akt dephosphorylation.
PMID:17488347 Erdogan A et al; J Thromb Haemost 5(8):1774-81 (2007)
... Apigenin inhibits the production of proinflammatory cytokines IL-1beta, IL-8, and TNF in LPS-stimulated human monocytes and mouse macrophages. The inhibitory effect on proinflammatory cytokine production persists even when apigenin is administered after LPS stimulation. Transient transfection experiments using NF-kappaB reporter constructs indicated that apigenin inhibits the transcriptional activity of NF-kappaB in LPS-stimulated mouse macrophages. The classical proteasome-dependent degradation of the NF-kappaB inhibitor IkappaBalpha was observed in apigenin LPS-stimulated human monocytes. Using EMSA ... apigenin does not alter NF-kappaB-DNA binding activity in human monocytes. Instead ... apigenin, as part of a non-canonical pathway, regulates NF-kappaB activity through hypophosphorylation of Ser536 in the p65 subunit and the inactivation of the IKK complex stimulated by LPS. The decreased phosphorylation on Ser536 observed in LPS-stimulated mouse macrophages treated with apigenin was overcome by the over-expression of IKKbeta. In addition ... /the/ studies indicate that apigenin inhibits in vivo LPS-induced TNF and the mortality induced by lethal doses of LPS. Collectively, these findings suggest a molecular mechanism by which apigenin suppresses inflammation and modulates the immune response in vivo.
PMID:17982104 Nicholas C et al; J Immunol 179(10):7121-7 (2007)
Treatment of /human prostate cancer/ LNCaP and PC-3 cells with apigenin causes G0-G1 phase arrest, decrease in total Rb protein and its phosphorylation at Ser780 and Ser807/811 in dose- and time-dependent fashion. Apigenin treatment caused increased phosphorylation of ERK1/2 and JNK1/2 and this sustained activation resulted in decreased ELK-1 phosphorylation and c-FOS expression thereby inhibiting cell survival. Use of kinase inhibitors induced ERK1/2 phosphorylation, albeit at different levels, and did not contribute to cell cycle arrest in comparison to apigenin treatment. Despite activation of MAPK pathway, apigenin caused a significant decrease in cyclin D1 expression that occurred simultaneously with the loss of Rb phosphorylation and inhibition of cell cycle progression. The reduced expression of cyclin D1 protein correlated with decrease in expression and phosphorylation of p38 and PI3K-Akt, which are regulators of cyclin D1 protein. Interestingly, apigenin caused a marked reduction in cyclin D1, D2 and E and their regulatory partners CDK 2, 4 and 6, operative in G0-G1 phase of the cell cycle. This was accompanied by a loss of RNA polymerase II phosphorylation, suggesting the effectiveness of apigenin in inhibiting transcription of these proteins. This study provides an insight into the molecular mechanism of apigenin in modulating various tyrosine kinases and perturbs cell cycle progression, suggesting its future development and use as anticancer agent in humans.
PMID:17457054 Shukla S, Gupta S; Cell Cycle 6(9):1102-14 (2007)
The aim of this study was to clarify the anti-inflammatory mechanism of apigenin. Apigenin inhibited the collagenase activity involved in rheumatoid arthritis (RA) and suppressed lipopolysaccharide (LPS)-induced nitric oxide (NO) production in a dose dependent manner in RAW 264.7 macrophage cells. Pretreatment with apigenin also attenuated LPS-induced cyclooxygenase-2 (COX-2) expression. In addition, apigenin profoundly reduced the tumor necrosis factor-alpha (TNF-alpha)-induced adhesion of monocytes to HUVEC monolayer. Apigenin significantly suppressed the TNF-alpha-stimulated upregulation of vascular cellular adhesion molecule-1 (VCAM-1)-, intracellular adhesion molecule-1 (ICAM-1)-, and E-selectin-mRNA to the basal levels. Taken together, these results suggest that apigenin has significant anti-inflammatory activity that involves blocking NO-mediated COX-2 expression and monocyte adherence ...
PMID:18038911 Lee JH et al; Arch Pharm Res 30(10):1318-27 (2007)
For more Mechanism of Action (Complete) data for APIGENIN (16 total), please visit the HSDB record page.
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
18
PharmaCompass offers a list of Apigenin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Apigenin manufacturer or Apigenin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Apigenin manufacturer or Apigenin supplier.
PharmaCompass also assists you with knowing the Apigenin API Price utilized in the formulation of products. Apigenin API Price is not always fixed or binding as the Apigenin Price is obtained through a variety of data sources. The Apigenin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Apigenin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Apigenin, including repackagers and relabelers. The FDA regulates Apigenin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Apigenin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Apigenin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Apigenin supplier is an individual or a company that provides Apigenin active pharmaceutical ingredient (API) or Apigenin finished formulations upon request. The Apigenin suppliers may include Apigenin API manufacturers, exporters, distributors and traders.
click here to find a list of Apigenin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Apigenin Drug Master File in Korea (Apigenin KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Apigenin. The MFDS reviews the Apigenin KDMF as part of the drug registration process and uses the information provided in the Apigenin KDMF to evaluate the safety and efficacy of the drug.
After submitting a Apigenin KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Apigenin API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Apigenin suppliers with KDMF on PharmaCompass.
Apigenin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Apigenin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Apigenin GMP manufacturer or Apigenin GMP API supplier for your needs.
A Apigenin CoA (Certificate of Analysis) is a formal document that attests to Apigenin's compliance with Apigenin specifications and serves as a tool for batch-level quality control.
Apigenin CoA mostly includes findings from lab analyses of a specific batch. For each Apigenin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Apigenin may be tested according to a variety of international standards, such as European Pharmacopoeia (Apigenin EP), Apigenin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Apigenin USP).
