
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
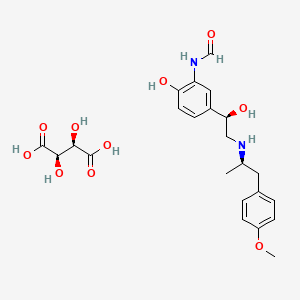

1. 200815-49-2
2. Brovana
3. (r,r)-arformoterol Tartrate
4. Arformoterol Tartrate [usan]
5. 5p8vj2i235
6. Arformoterol Tartrate (usan)
7. Formamide, N-[2-hydroxy-5-[(1r)-1-hydroxy-2-[[(1r)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]-, (2r,3r)-2,3-dihydroxybutanedioate (1:1)
8. (r,r)-formoterol Tartrate
9. (-)-n-(2-hydroxy-5-((1r)-1-hydroxy-2-(((1r)-2-(4-methoxyphenyl)-1-methylethyl)amino)ethyl)phenyl)formamide Hydrogen (2r,3r)-2,3-dihydroxybutanedioate (salt)
10. Formamide, N-(2-hydroxy-5-((1r)-1-hydroxy-2-(((1r)-2-(4-methoxyphenyl)-1-methylethyl)amino)ethyl)phenyl)-, (2r,3r)-2,3-dihydroxybutanedioate (1:1) (salt)
11. N-[2-hydroxy-5-[(1r)-1-hydroxy-2-[[(1r)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]-formamide (2r,3r)-2,3-dihydroxybutanedioate (1:1) (salt)
12. Brovana Inhalation Solution
13. Unii-5p8vj2i235
14. Brovana (tn)
15. Arformotero Ltartrate
16. Dtxsid80173903
17. Arformoterol Tartrate [vandf]
18. S5217
19. Arformoterol Tartrate [mart.]
20. Akos005145740
21. Arformoterol Tartrate [who-dd]
22. Ccg-269651
23. Arformoterol Tartrate, >=98% (hplc)
24. Ba171831
25. Bs-42158
26. Arformoterol Tartrate [orange Book]
27. Formoterol R,r-form L-tartrate [mi]
28. D02981
29. 815f492
30. Q-101035
31. Q27262686
32. (2r,3r)-2,3-dihydroxybutanedioic Acid;n-[2-hydroxy-5-[(1r)-1-hydroxy-2-[[(2r)-1-(4-methoxyphenyl)propan-2-yl]amino]ethyl]phenyl]formamide
33. (r,r)-formoterol Tartrate; N-(2-hydroxy-5-((1r)-1-hydroxy-2-(((1r)-2-(4-methoxyphenyl)-1-methylethyl)amino)ethyl)phenyl)formamide (2r,3r)-2,3-dihydroxybutanedioate (1:1)
34. [(2r)-2-(3-formamido-4-hydroxyphenyl)-2-hydroxyethyl]-[(2r)-1-(4-methoxyphenyl)propan-2-yl]azanium;(2r,3r)-2,3,4-trihydroxy-4-oxobutanoate
35. N-[2-hydroxy-5-[(1r)-1-hydroxy-2-[[(1r)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]formamide (2r,3r)-2,3-dihydroxybutane Dioate
36. N-[2-hydroxy-5-[(1r)-1-hydroxy-2-[[(1r)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]formamide (2r,3r)-2,3-dihydroxybutanedioate
37. N-[2-hydroxy-5-[(1r)-1-hydroxy-2-[[(1r)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]formamide L-tartrate
| Molecular Weight | 494.5 g/mol |
|---|---|
| Molecular Formula | C23H30N2O10 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 11 |
| Exact Mass | 494.19004516 g/mol |
| Monoisotopic Mass | 494.19004516 g/mol |
| Topological Polar Surface Area | 206 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 521 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Brovana |
| PubMed Health | Arformoterol (By breathing) |
| Drug Classes | Bronchodilator |
| Active Ingredient | Arformoterol tartrate |
| Dosage Form | Solution |
| Route | Inhalation |
| Strength | eq 0.015mg base/2ml |
| Market Status | Prescription |
| Company | Sunovion |
| 2 of 2 | |
|---|---|
| Drug Name | Brovana |
| PubMed Health | Arformoterol (By breathing) |
| Drug Classes | Bronchodilator |
| Active Ingredient | Arformoterol tartrate |
| Dosage Form | Solution |
| Route | Inhalation |
| Strength | eq 0.015mg base/2ml |
| Market Status | Prescription |
| Company | Sunovion |
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2019-04-12
Pay. Date : 2018-07-17
DMF Number : 32990
Submission : 2018-07-25
Status : Active
Type : II

NDC Package Code : 64567-0020
Start Marketing Date : 2018-07-19
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Samoh Pharmaceutical Co., Ltd.
Registration Date : 2022-03-24
Registration Number : 20220324-211-J-1236
Manufacturer Name : INKE, SA
Manufacturer Address : C/ Argent 1, Area industrial del Llobregat 08755, Castellbisbal (Barcelona) Spain

| Available Reg Filing : CN |

GDUFA
DMF Review : Reviewed
Rev. Date : 2022-07-14
Pay. Date : 2022-03-17
DMF Number : 36435
Submission : 2022-05-16
Status : Active
Type : II

NDC Package Code : 64567-0020
Start Marketing Date : 2018-07-19
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Samoh Pharmaceutical Co., Ltd.
Registration Date : 2022-03-24
Registration Number : 20220324-211-J-1236
Manufacturer Name : INKE, SA
Manufacturer Address : C/ Argent 1, Area industrial del Llobregat 08755, Castellbisbal (Barcelona) Spain

| Available Reg Filing : CN |

GDUFA
DMF Review : Reviewed
Rev. Date : 2018-10-24
Pay. Date : 2018-07-26
DMF Number : 28013
Submission : 2014-03-15
Status : Active
Type : II

Date of Issue : 2022-06-17
Valid Till : 2025-07-14
Written Confirmation Number : WC-0021n
Address of the Firm :

NDC Package Code : 14501-0077
Start Marketing Date : 2012-07-02
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2015-06-09
Pay. Date : 2015-03-27
DMF Number : 28868
Submission : 2015-03-27
Status : Active
Type : II

NDC Package Code : 66039-909
Start Marketing Date : 2015-03-27
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2014-05-15
Pay. Date : 2014-05-02
DMF Number : 22968
Submission : 2009-08-19
Status : Active
Type : II

Date of Issue : 2022-06-08
Valid Till : 2025-09-08
Written Confirmation Number : WC-0144
Address of the Firm :

NDC Package Code : 53104-7731
Start Marketing Date : 2017-01-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


Date of Issue : 2022-07-28
Valid Till : 2025-07-02
Written Confirmation Number : WC-0227
Address of the Firm :



Date of Issue : 2020-01-28
Valid Till : 2022-09-08
Written Confirmation Number : WC-0144A5
Address of the Firm :

NDC Package Code : 53104-7731
Start Marketing Date : 2017-01-01
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
GDUFA
DMF Review : Complete
Rev. Date : 2022-07-14
Pay. Date : 2022-03-17
DMF Number : 36435
Submission : 2022-05-16
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2019-04-12
Pay. Date : 2018-07-17
DMF Number : 32990
Submission : 2018-07-25
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2014-05-15
Pay. Date : 2014-05-02
DMF Number : 22968
Submission : 2009-08-19
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2015-06-09
Pay. Date : 2015-03-27
DMF Number : 28868
Submission : 2015-03-27
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2018-10-24
Pay. Date : 2018-07-26
DMF Number : 28013
Submission : 2014-03-15
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Date of Issue : 2022-06-08
Valid Till : 2025-09-08
Written Confirmation Number : WC-0144
Address of the Firm : Plot. D-7 MIDC, Kurkumbh, Dist. Pune-413802

Date of Issue : 2020-01-28
Valid Till : 2022-09-08
Written Confirmation Number : WC-0144A5
Address of the Firm : Plot. D-7 MIDC, Kurkumbh, Dist. Pune-413802

Date of Issue : 2022-07-28
Valid Till : 2025-07-02
Written Confirmation Number : WC-0227
Address of the Firm : Plot No. J-73, MIDC, Tarapur, Boisar, Dist- Palghar 401506, Maharashtra, India

Date of Issue : 2022-06-17
Valid Till : 2025-07-14
Written Confirmation Number : WC-0021n
Address of the Firm : Sy. Nos: 317, 320, 321, 322, 323, 604 & 605, Rudraram (Village), Patancheru (Man...

Date of Issue : 2019-07-15
Valid Till : 2022-07-14
Written Confirmation Number : WC-0021
Address of the Firm : Sy. No.317&323, Rudraram Village, Patancheru Mandal, Sangaredy District-502329 T...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The acquisition of Brovana® (arformoterol tartrate) Inhalation Solution and Xopenex HFA® (levalbuterol tartrate) Inhalation Aerosol brands expands Lupin’s portfolio of inhalation products in the U.S. and strengthens the company’s presence in the respiratory therapy area.
Lead Product(s): Arformoterol Tartrate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Brovana
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Lupin Ltd
Deal Size: $75.0 million Upfront Cash: Undisclosed
Deal Type: Acquisition October 20, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Arformoterol Tartrate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Lupin Ltd
Deal Size : $75.0 million
Deal Type : Acquisition
Lupin Signs Agreement to Acquire Two Inhalation Brands from Sunovion
Details : The acquisition of Brovana® (arformoterol tartrate) Inhalation Solution and Xopenex HFA® (levalbuterol tartrate) Inhalation Aerosol brands expands Lupin’s portfolio of inhalation products in the U.S. and strengthens the company’s presence in the re...
Brand Name : Brovana
Molecule Type : Small molecule
Upfront Cash : Undisclosed
October 20, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Arformoterol Tartrate inhalation solution, EQ 0.015 mg Base/2 ml is indicated for the long-term, twice daily (morning and evening) maintenance treatment of bronchoconstriction in patients with COPD, including chronic bronchitis and emphysema.
Lead Product(s): Arformoterol Tartrate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Arformoterol Tartrate-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 24, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Arformoterol Tartrate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Arformoterol Tartrate inhalation solution, EQ 0.015 mg Base/2 ml is indicated for the long-term, twice daily (morning and evening) maintenance treatment of bronchoconstriction in patients with COPD, including chronic bronchitis and emphysema.
Brand Name : Arformoterol Tartrate-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
August 24, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Cipla’s Arformoterol Tartrate Inhalation Solution 15 mcg / 2 mL is AN-rated generic therapeutic equivalent version of Sunovion Pharmaceuticals Inc.’s Brovana®.
Lead Product(s): Arformoterol Tartrate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Undisclosed
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 23, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Arformoterol Tartrate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Cipla Receives Final Approval for Generic Version Of Sunovion Pharmaceuticals, Inc.'s Brovana®
Details : Cipla’s Arformoterol Tartrate Inhalation Solution 15 mcg / 2 mL is AN-rated generic therapeutic equivalent version of Sunovion Pharmaceuticals Inc.’s Brovana®.
Brand Name : Undisclosed
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 23, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Arformoterol Tartrate Inhalation Solution 15mcg/2mL, is indicated for the long-term, twice daily maintenance treatment of bronchoconstriction in patients with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema. It is for use by nebulization only.
Lead Product(s): Arformoterol Tartrate
Therapeutic Area: Pulmonary/Respiratory Diseases Brand Name: Undisclosed
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 03, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Arformoterol Tartrate
Therapeutic Area : Pulmonary/Respiratory Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Lupin Launches Authorized Generic Version of Brovana® in the United States
Details : Arformoterol Tartrate Inhalation Solution 15mcg/2mL, is indicated for the long-term, twice daily maintenance treatment of bronchoconstriction in patients with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema. It is for us...
Brand Name : Undisclosed
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 03, 2021

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results](R)-N-(2-(Benzyloxy)-5-(2-bromo-1-hydroxyethyl)phe...
CAS Number : 201677-59-0
End Use API : Arformoterol Tartrate
About The Company : Hiray Pharma Solutions is an international end-to-end CDMO, facilitating the development and manufacturing of important drug products and key intermediates arou...

(R)-N-Benzyl-1-(4-methoxyphenyl)propan-2-amine (S)...
CAS Number : 188690-84-8
End Use API : Arformoterol Tartrate
About The Company : Hiray Pharma Solutions is an international end-to-end CDMO, facilitating the development and manufacturing of important drug products and key intermediates arou...

N-[5-bromo-2-(2-nitrophenyl)sulfanyl-phenyl]formam...
CAS Number : 201677-59-0
End Use API : Arformoterol Tartrate
About The Company : With nearly seven decades of manufacturing expertise, Speichim Processing actively formulates circular economy strategies by reclaiming waste and chemical produ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
Regulatory Info : RX
Registration Country : USA
Brand Name : ARFORMOTEROL TARTRATE
Dosage Form : SOLUTION;INHALATION
Dosage Strength : EQ 0.015MG BASE/2ML
Packaging :
Approval Date : 2024-06-03
Application Number : 215032
Regulatory Info : RX
Registration Country : USA
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ARFORMOTEROL TARTRATE
Dosage Form : SOLUTION;INHALATION
Dosage Strength : EQ 0.015MG BASE/2ML
Packaging :
Approval Date : 2024-04-02
Application Number : 214901
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ARFORMOTEROL TARTRATE
Dosage Form : SOLUTION;INHALATION
Dosage Strength : EQ 0.015MG BASE/2ML
Packaging :
Approval Date : 2022-05-10
Application Number : 214779
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ARFORMOTEROL TARTRATE
Dosage Form : SOLUTION;INHALATION
Dosage Strength : EQ 0.015MG BASE/2ML
Packaging :
Approval Date : 2021-06-22
Application Number : 213762
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ARFORMOTEROL TARTRATE
Dosage Form : SOLUTION;INHALATION
Dosage Strength : EQ 0.015MG BASE/2ML
Packaging :
Approval Date : 2024-05-17
Application Number : 216303
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : BROVANA
Dosage Form : SOLUTION;INHALATION
Dosage Strength : EQ 0.015MG BASE/2ML
Packaging :
Approval Date : 2006-10-06
Application Number : 21912
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ARFORMOTEROL TARTRATE
Dosage Form : SOLUTION;INHALATION
Dosage Strength : EQ 0.015MG BASE/2ML
Packaging :
Approval Date : 2022-02-07
Application Number : 213068
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ARFORMOTEROL TARTRATE
Dosage Form : SOLUTION;INHALATION
Dosage Strength : EQ 0.015MG BASE/2ML
Packaging :
Approval Date : 2022-11-15
Application Number : 216128
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ARFORMOTEROL TARTRATE
Dosage Form : SOLUTION;INHALATION
Dosage Strength : EQ 0.015MG BASE/2ML
Packaging :
Approval Date : 2021-11-09
Application Number : 200293
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : ARFORMOTEROL TARTRATE
Dosage Form : SOLUTION;INHALATION
Dosage Strength : EQ 0.015MG BASE/2ML
Packaging :
Approval Date : 2022-03-02
Application Number : 214736
Regulatory Info : RX
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Dosage Form : Injectable / Parenteral
Grade : Parenteral
Brand Name : NaCl Multi-compendial Low...
Application : Parenteral
Excipient Details : A & C's Sodium Chloride multi-compendial low endotoxin is an excipient meeting USP-NF, EP, BP and JP monographs.
Pharmacopoeia Ref : Multi-compendial
Technical Specs : Low Endotoxin
Ingredient(s) : Sodium Chloride Excipient
Dosage Form : Injectable / Parenteral
Grade : Parenteral
Brand Name : Sodium Chloride USP
Application : Parenteral
Excipient Details : A & C's Sodium Chloride is an excipient meeting the USP monograph.
Dosage Form : Tablet
Grade : Oral
Brand Name : Sodium Citrate Dihydrate ...
Application : Controlled & Modified Release
Excipient Details : A & C's Sodium Citrate Dihydrate multi-compendial is a trisodium salt of citric acid.
Pharmacopoeia Ref : Multi-compendial
Technical Specs : Not Available
Ingredient(s) : Sodium Citrate Dihydrate Excipient
Dosage Form : Tablet
Grade : Oral
Brand Name : Sodium Citrate Dihydrate ...
Application : Controlled & Modified Release
Excipient Details : A & C's Sodium Citrate Dihydrate USP-NF is a trisodium salt of citric acid.
Pharmacopoeia Ref : USP/NF
Technical Specs : Not Available
Ingredient(s) : Sodium Citrate Dihydrate Excipient
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Brand Name : Sodium Chloride USP
Application : Parenteral
Excipient Details : A & C's Sodium Chloride is an excipient meeting the USP monograph.
Brand Name : NaCl Multi-compendial Low Endotoxin
Application : Parenteral
Excipient Details : A & C's Sodium Chloride multi-compendial low endotoxin is an excipient meeting USP-NF, EP, BP and JP monographs.
Pharmacopoeia Ref : Multi-compendial
Technical Specs : Low Endotoxin
Ingredient(s) : Sodium Chloride Excipient
Global Sales Information

Market Place

Reply
26 Jan 2022
Reply
15 Jun 2021
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
REF. STANDARDS & IMPURITIES

CAS Number : 1795135-61-3
Quantity Per Vial :
Sale Unit :
Price :
Details : In stock
Monograph :
Storage :
Code/Batch No : A0131.01

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?