
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Australia

DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
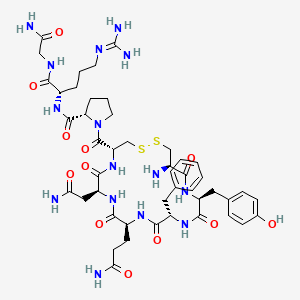

1. Arg Vasopressin
2. Arg-vasopressin
3. Arginine Vasopressin
4. Argipressin Tannate
5. Vasopressin, Arginine
1. Arginine Vasopressin
2. 113-79-1
3. Argipressine
4. Pitressin
5. Beta-hypophamine
6. 8-arginine-vasopressin
7. Argipressin Tannate
8. Vasopressin (arginine Form)
9. Arginine-vasopressin
10. Chebi:34543
11. Vasophysin
12. Arg-vasopressin
13. Chembl373742
14. (arg8)-vasopressin
15. [arg8]-vasopressin
16. (2s)-1-[(4r,7s,10s,13s,16s,19r)-19-amino-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-13-benzyl-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]-n-[(2s)-1-[(2-amino-2-oxoethyl)amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]pyrrolidine-2-carboxamide
17. 8-l-arginine Vasopressin
18. Argipresina
19. Argipressina
20. Argipressinum
21. Avp
22. Arg8-vasopressin
23. Argipressin [inn]
24. Argipressina [dcit]
25. Ncgc00166306-01
26. Argipressin [inn:ban]
27. 8-l-arginine-vasopressin
28. Argipresina [inn-spanish]
29. Argipressine [inn-french]
30. Argipressinum [inn-latin]
31. Unii-y4907o6mfd
32. Rindervasopressin
33. [3h]vasopressin
34. Einecs 204-035-4
35. 3-(phenylalanine)-8-arginineoxytocin
36. Arg8-vasopressin;avp
37. Arginine-8-vasopressin
38. Argipressin Or Lypressin
39. [8-arginine]vasopressin
40. [3h]argipressin Tannate
41. Antidiuretic Hormone (adh)
42. Arginine Vasopressin (avp)
43. Argipressin Tannate [usan]
44. Arginine Antidiuretic Hormone
45. Dsstox_cid_28324
46. Dsstox_rid_82752
47. Dsstox_gsid_48349
48. Schembl43139
49. Vasopressin, 8-l-arginine-
50. [cyclo S-s]cyfqncprg-nh2
51. Gtpl2168
52. Y4907o6mfd
53. Dtxsid0048349
54. Schembl17874853
55. Bdbm35667
56. Tox21_113037
57. Bdbm50044777
58. Mfcd00076738
59. Ncgc00166306-02
60. Ncgc00188439-01
61. Cas-113-79-1
62. H-cys-tyr-phe-gln-asn-cys-pro-arg-gly-nh2
63. Oxytocin, 3-(l-phenylalanine)-8-l-arginine-
64. Vasopressin, 8-l-arginine- (7ci,8ci,9ci)
65. Roxybenzyl-6,9,12,15,18-pentaoxo- (6ci)
66. 113a791
67. Q183011
68. Cys-tyr-phe-gln-asn-cys-pro-arg-gly-nh2[disulfide Bridge: 1-6]
69. (2s)-1-[(4r,7s,10s,13s,16s,19r)-19-amino-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-16-[(4-hydroxyphenyl)methyl]-6,9,12,15,18-pentaoxo-13-(phenylmethyl)1,2-dithia-5,8,11,14,17-pentazacycloicosane-4-carbonyl]-n-[(2s)-1-[(2-amino-2-oxoethyl)amino]-5-(diaminomethylideneamino)-1-oxopentan-2-yl]pyrrolidine-2-carboxamide
70. 1,2-dithia-5,8,11,14,17-pentaazacycloeicosane-10-propionamide, 19-amino-13-benzyl-7-(carbamoylmethyl)-4-[2-[[1-[(carbamoylmethyl)carbamoyl]-4-guanidinobutyl]carbamoyl]-1-pyrrolidinylcarbonyl]-16-p-hyd
71. 1-{[(4r,7s,10s,13s,16s,19r)-19-amino-7-(2-amino-2-oxoethyl)-10-(3-amino-3-oxopropyl)-13-benzyl-16-(4-hydroxybenzyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosan-4-yl]carbonyl}-l-prolyl-l-arginylglycinamide
72. Glycinamide, L-cysteinyl-l-tyrosyl-l-phenylalanyl-l-glutaminyl-l-asparaginyl-l-cysteinyl-l-prolyl-l-arginyl-, Cyclic (1>6)-disulfide
| Molecular Weight | 1084.2 g/mol |
|---|---|
| Molecular Formula | C46H65N15O12S2 |
| XLogP3 | -4.8 |
| Hydrogen Bond Donor Count | 14 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 19 |
| Exact Mass | 1083.43785492 g/mol |
| Monoisotopic Mass | 1083.43785492 g/mol |
| Topological Polar Surface Area | 515 Ų |
| Heavy Atom Count | 75 |
| Formal Charge | 0 |
| Complexity | 2070 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Vasoconstrictor Agents
Drugs used to cause constriction of the blood vessels. (See all compounds classified as Vasoconstrictor Agents.)
Antidiuretic Agents
Agents that reduce the excretion of URINE, most notably the octapeptide VASOPRESSINS. (See all compounds classified as Antidiuretic Agents.)
Hemostatics
Agents acting to arrest the flow of blood. Absorbable hemostatics arrest bleeding either by the formation of an artificial clot or by providing a mechanical matrix that facilitates clotting when applied directly to the bleeding surface. These agents function more at the capillary level and are not effective at stemming arterial or venous bleeding under any significant intravascular pressure. (See all compounds classified as Hemostatics.)
 Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.
Bachem Group is a public, innovation-driven company specializing in the development and manufacturing of peptides and oligonucleotides.
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2017-09-29
Pay. Date : 2017-08-24
DMF Number : 28532
Submission : 2014-08-01
Status : Active
Type : II

NDC Package Code : 52416-127
Start Marketing Date : 2019-05-16
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT FOR HUMAN PRESCRIPTION COMPOUNDING
 Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.

GDUFA
DMF Review : Reviewed
Rev. Date : 2018-09-11
Pay. Date : 2018-08-27
DMF Number : 33119
Submission : 2018-08-27
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2018-03-02
Pay. Date : 2017-10-04
DMF Number : 32116
Submission : 2017-10-03
Status : Active
Type : II

NDC Package Code : 63586-0004
Start Marketing Date : 2023-01-02
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (100g/100g)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2018-07-13
Pay. Date : 2018-05-04
DMF Number : 32769
Submission : 2018-05-05
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2018-03-06
Pay. Date : 2017-11-27
DMF Number : 32146
Submission : 2017-10-12
Status : Active
Type : II

NDC Package Code : 41701-008
Start Marketing Date : 2016-03-28
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (0.3g/.3g)
Marketing Category : BULK INGREDIENT


GDUFA
DMF Review : Reviewed
Rev. Date : 2023-01-19
Pay. Date : 2022-11-25
DMF Number : 37634
Submission : 2022-12-03
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 25736
Submission : 2012-01-24
Status : Active
Type : II

NDC Package Code : 35207-0005
Start Marketing Date : 2012-07-25
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1g/g)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 27577
Submission : 2013-12-19
Status : Inactive
Type : II

NDC Package Code : 63586-0004
Start Marketing Date : 2023-01-02
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (100g/100g)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
About the Company : HRV Global is a leading global manufacturer, seller & exporter of a wide range of APIs, advanced intermediates, pellets, food grade chemicals, food additives & food ingredients. It...
 Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
Omgene: R&D-based biopharmaceutical company with GMP facilities, focused on innovation and high-quality, affordable medicines.
About the Company : Omgene Life Sciences Private Limited is an R&D-driven biopharmaceutical company specializing in biopharmaceuticals, peptides, semi-synthetic, and synthetic actives. As a vertically...
About the Company : Founded in 1986 by Mr. P.V. Ramaprasad Reddy, Mr. K. Nityananda Reddy and a small group of highly committed professionals, Aurobindo Pharma was born off a vision. The company comme...

About the Company : BCN Peptides is a privately own company completely focused on the cGMP manufacture of Bioactive Peptides for Pharmaceutical and Veterinary applications. We are a customer oriented...

About the Company : JIN DUN Medical Research Institute is affiliated to Shanghai JIN DUN Industrial Co., Ltd., headquartered in Shanghai, adjacent to Hongqiao High-speed Railway Station and Hongqiao I...

About the Company : Piramal Pharma Solutions (PPS) is a CDMO that provides end-to-end solutions for drug development and manufacturing across the drug life cycle to its clients in North America, Europ...

About the Company : Shenzhen JYMed Technology Co.,Ltd is a high-tech enterprise engaged in research and development, manufacturing and commercialization of peptides based products, including active ph...

About the Company : With over a decade of experience in the Pharmaceutical and Health sector, HNB stands as a contemporary company specializing in the exploration, advancement, manufacturing, processi...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Details:
The launch of authorized generic version of Par Pharmaceutical’s VASOSTRICT® (vasopressin injection, USP) Vials in the U.S. Market was approved by the U.S. Food and Drug Administration (USFDA).
Lead Product(s): Arginine Vasopressin
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Vasopressin-Generic
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable February 09, 2022
Lead Product(s) : Arginine Vasopressin
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Dr. Reddy's Laboratories Announces the Launch of Its Authorized Generic Version of VASOSTRICT® (v...
Details : The launch of authorized generic version of Par Pharmaceutical’s VASOSTRICT® (vasopressin injection, USP) Vials in the U.S. Market was approved by the U.S. Food and Drug Administration (USFDA).
Brand Name : Vasopressin-Generic
Molecule Type : Peptide
Upfront Cash : Not Applicable
February 09, 2022
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vasopressin injection is indicated to increase blood pressure in adults with vasodilatory shock who remain hypotensive despite fluids and catecholamines and Vasostrict is a RFLD.
Lead Product(s): Arginine Vasopressin
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Vasostrict-Generic
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 14, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Arginine Vasopressin
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Fresenius Kabi Launches Vasopressin Injection, USP Expanding U.S. Critical Care Portfolio
Details : Vasopressin injection is indicated to increase blood pressure in adults with vasodilatory shock who remain hypotensive despite fluids and catecholamines and Vasostrict is a RFLD.
Brand Name : Vasostrict-Generic
Molecule Type : Peptide
Upfront Cash : Not Applicable
June 14, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vasopressin Injection is indicated to increase blood pressure in adults with vasodilatory shocks, such as due to post-cardiotomy or sepsis, and remain hypotensive despite fluids and catecholamines.
Lead Product(s): Arginine Vasopressin
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Vasopressin-Generic
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 16, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Arginine Vasopressin
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Aurobindo Pharma Arm Gets Final US FDA Nod for Vasopressin injection
Details : Vasopressin Injection is indicated to increase blood pressure in adults with vasodilatory shocks, such as due to post-cardiotomy or sepsis, and remain hypotensive despite fluids and catecholamines.
Brand Name : Vasopressin-Generic
Molecule Type : Peptide
Upfront Cash : Not Applicable
August 16, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vasopressin injection 1mL is a generic version of the antidiuretic hormone Vasostrict® is indicated to increase blood pressure in adults with vasodilatory shock who remain hypotensive despite fluids and catecholamines.
Lead Product(s): Arginine Vasopressin
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Vasopressin-Generic
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 11, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Arginine Vasopressin
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Amneal Launches 4 New Generic Products, Including Vasopressin Single-dose
Details : Vasopressin injection 1mL is a generic version of the antidiuretic hormone Vasostrict® is indicated to increase blood pressure in adults with vasodilatory shock who remain hypotensive despite fluids and catecholamines.
Brand Name : Vasopressin-Generic
Molecule Type : Peptide
Upfront Cash : Not Applicable
August 11, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
In August, the U.S. District Court for the District of Delaware held that Eagle’s proposed vasopressin product does not infringe any of the patents Par Pharmaceutical, Inc. et al. asserted against the Company.
Lead Product(s): Arginine Vasopressin
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Vasopressin-Generic
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 29, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Arginine Vasopressin
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Eagle Pharmaceuticals Receives 180 Days of Marketing Exclusivity for Recently Approved Vasopressin
Details : In August, the U.S. District Court for the District of Delaware held that Eagle’s proposed vasopressin product does not infringe any of the patents Par Pharmaceutical, Inc. et al. asserted against the Company.
Brand Name : Vasopressin-Generic
Molecule Type : Peptide
Upfront Cash : Not Applicable
December 29, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The U.S. District Court for the District of Delaware issue a temporary restraining order and preliminary injunction preventing the launch of Eagle’s recently approved vasopressin product.
Lead Product(s): Arginine Vasopressin
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Undisclosed
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Par Pharmaceutical
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 29, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Arginine Vasopressin
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Par Pharmaceutical
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : The U.S. District Court for the District of Delaware issue a temporary restraining order and preliminary injunction preventing the launch of Eagle’s recently approved vasopressin product.
Brand Name : Undisclosed
Molecule Type : Peptide
Upfront Cash : Not Applicable
December 29, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
This approval follows the recent U.S. District Court for the District of Delaware decision holding that Eagle’s proposed vasopressin product does not infringe any of the patents Par Pharmaceutical, Inc. et al. asserted against the Company.
Lead Product(s): Arginine Vasopressin
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Vasopressin-Generic
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable December 15, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Arginine Vasopressin
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Eagle Pharmaceuticals Receives FDA Approval for Vasopressin
Details : This approval follows the recent U.S. District Court for the District of Delaware decision holding that Eagle’s proposed vasopressin product does not infringe any of the patents Par Pharmaceutical, Inc. et al. asserted against the Company.
Brand Name : Vasopressin-Generic
Molecule Type : Peptide
Upfront Cash : Not Applicable
December 15, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Eagle is first to file an Abbreviated New Drug Application (“ANDA”) referencing Vasostrict®, which had total U.S. sales of $786 million in 2020.
Lead Product(s): Arginine Vasopressin
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Undisclosed
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 31, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Arginine Vasopressin
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Eagle Pharmaceuticals Wins Vasopressin Patent Trial
Details : Eagle is first to file an Abbreviated New Drug Application (“ANDA”) referencing Vasostrict®, which had total U.S. sales of $786 million in 2020.
Brand Name : Undisclosed
Molecule Type : Peptide
Upfront Cash : Not Applicable
August 31, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
United States District Court for the District of Delaware's non-infringement found non-infringement of Endo's patents by Eagle's proposed ANDA for Par Sterile Products, LLC's VASOSTRICT® product, it did not rule on the patents' validity.
Lead Product(s): Arginine Vasopressin
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Vasostrict
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 31, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Arginine Vasopressin
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Endo Comments on Non-Infringement Ruling in VASOSTRICT® Patent Litigation
Details : United States District Court for the District of Delaware's non-infringement found non-infringement of Endo's patents by Eagle's proposed ANDA for Par Sterile Products, LLC's VASOSTRICT® product, it did not rule on the patents' validity.
Brand Name : Vasostrict
Molecule Type : Peptide
Upfront Cash : Not Applicable
August 31, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The U.S. FDA has issued a complete response letter for Eagle's Abbreviated New Drug Application for vasopressin. Eagle has now had two conversations with FDA regarding the CRL and will have an additional meeting with FDA within 30 days.
Lead Product(s): Arginine Vasopressin
Therapeutic Area: Cardiology/Vascular Diseases Brand Name: Undisclosed
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable February 02, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Arginine Vasopressin
Therapeutic Area : Cardiology/Vascular Diseases
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : The U.S. FDA has issued a complete response letter for Eagle's Abbreviated New Drug Application for vasopressin. Eagle has now had two conversations with FDA regarding the CRL and will have an additional meeting with FDA within 30 days.
Brand Name : Undisclosed
Molecule Type : Peptide
Upfront Cash : Not Applicable
February 02, 2021

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
DRL offers a portfolio of products & services, including APIs, CMO services, generics, biosimilars & differentiated formulations.
RLD : No
TE Code : AP
Brand Name : VASOPRESSIN
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 20UNITS/ML (20UNITS/ML)
Approval Date : 2024-05-08
Application Number : 213988
RX/OTC/DISCN : RX
RLD : No
TE Code : AP
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : VASOPRESSIN
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 200UNITS/10ML(20UNITS/ML)
Approval Date :
Application Number : 212945
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : VASOPRESSIN
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 20UNITS/ML (20UNITS/ML)
Approval Date : 2022-07-18
Application Number : 211857
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : VASOPRESSIN
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 20UNITS/ML (20UNITS/ML)
Approval Date : 2022-08-15
Application Number : 214314
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : VASOPRESSIN IN SODIUM CHLORIDE 0.9%
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 20UNITS/100ML (0.2UNITS/ML)
Approval Date : 2023-09-29
Application Number : 217569
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : VASOPRESSIN
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 20UNITS/ML (20UNITS/ML)
Approval Date : 2021-12-15
Application Number : 211538
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : VASOPRESSIN
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 20UNITS/ML (20UNITS/ML)
Approval Date : 2023-05-26
Application Number : 213206
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AP
Brand Name : VASOPRESSIN
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 20UNITS/ML (20UNITS/ML)
Approval Date : 2024-02-09
Application Number : 216963
RX/OTC/DISCN : RX
RLD : No
TE Code : AP

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : PITRESSIN TANNATE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 5PRESSOR UNITS/ML **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Approval Date : 1982-01-01
Application Number : 3402
RX/OTC/DISCN : DISCN
RLD : Yes
TE Code :

Brand Name : VASOSTRICT
Dosage Form : SOLUTION;INTRAVENOUS
Dosage Strength : 20UNITS/100ML (0.2UNITS/ML)
Approval Date : 2021-04-21
Application Number : 204485
RX/OTC/DISCN : RX
RLD : Yes
TE Code : AP

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Ukraine
Brand Name : Projecta
Dosage Form : Solution for Injection
Dosage Strength : 20 VUML
Packaging : 1ML
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Ukraine

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Embesin
Dosage Form : Concentrate for infusion solution, solution
Dosage Strength : 20 IE/ml
Packaging : Ampoule av glass 10 2ml
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : VASOPRESSIN INJECTION, USP
Dosage Form : SOLUTION
Dosage Strength : 20UNIT/ML
Packaging : 1ML
Approval Date :
Application Number : 2139502
Regulatory Info : Prescription
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Canada
Brand Name : VASOPRESSIN INJECTION USP
Dosage Form : SOLUTION
Dosage Strength : 20UNIT/ML
Packaging : 2/5ML
Approval Date :
Application Number : 2247938
Regulatory Info : Prescription
Registration Country : Canada

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : POR 8
Dosage Form : INJ
Dosage Strength : 5IU
Packaging : 1X5IU
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 20UNIT
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info : Generic
Dosage : Injection
Dosage Strength : 20UNIT
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Korea
Brand Name : HANLIM VASOPRESSIN
Dosage Form : INJECTION
Dosage Strength : 20MG
Packaging : 1mL,10A
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Korea

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : 1mL,10A
Regulatory Info : Generic
Dosage : INJECTION
Dosage Strength : 20MG
Brand Name : HANLIM VASOPRESSIN
Approval Date :
Application Number :
Registration Country : South Korea

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Vial
Dosage Strength : 20IU
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Vial
Dosage Strength : 20IU
Brand Name :
Approval Date :
Application Number :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Turkey
Brand Name :
Dosage Form : Injectable
Dosage Strength : 0.2IU/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Turkey

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Injectable
Dosage Strength : 0.2IU/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : Turkey

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Turkey
Brand Name :
Dosage Form : Injectable
Dosage Strength : 0.4IU/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Turkey

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Injectable
Dosage Strength : 0.4IU/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : Turkey

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Turkey
Brand Name :
Dosage Form : Injectable
Dosage Strength : 0.6IU/ML
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Turkey

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging :
Regulatory Info :
Dosage : Injectable
Dosage Strength : 0.6IU/ML
Brand Name :
Approval Date :
Application Number :
Registration Country : Turkey

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Dosage Form : Concentrate for infusion solutio...
Dosage Strength : 20 IE/ml
Price Per Pack (Euro) : 643.06
Published in :
Country : Norway
RX/OTC/DISCN :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Main Therapeutic Indication : Nephrology
Currency : USD
2020 Revenue in Millions : 786
2019 Revenue in Millions : 532
Growth (%) : 48

Main Therapeutic Indication : Nephrology
Currency : USD
2021 Revenue in Millions : 902
2020 Revenue in Millions : 786
Growth (%) : 15

Main Therapeutic Indication : Cardiology/Vascular Diseases
Currency : USD
2022 Revenue in Millions : 254
2021 Revenue in Millions : 902
Growth (%) : -72

Main Therapeutic Indication : Cardiology/Vascular Diseases
Currency : USD
2023 Revenue in Millions : 93
2022 Revenue in Millions : 254
Growth (%) : -63

Main Therapeutic Indication : Renal Disorders
Currency : USD
2018 Revenue in Millions : 454
2017 Revenue in Millions : 400
Growth (%) : 14%

Main Therapeutic Indication : Nephrology
Currency : USD
2019 Revenue in Millions : 532
2018 Revenue in Millions : 454
Growth (%) : 17

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Patents & EXCLUSIVITIES
Patent Expiration Date : 2035-01-30
US Patent Number : 9925233
Drug Substance Claim :
Drug Product Claim :
Application Number : 204485
Patent Use Code : U-1857
Delist Requested :
Patent Use Description : TO INCREASE BLOOD PRES...
Patent Expiration Date : 2035-01-30

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Patent Expiration Date : 2035-01-30
US Patent Number : 9968649
Drug Substance Claim :
Drug Product Claim :
Application Number : 204485
Patent Use Code : U-1857
Delist Requested :
Patent Use Description : TO INCREASE BLOOD PRES...
Patent Expiration Date : 2035-01-30

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Patent Expiration Date : 2035-01-30
US Patent Number : 9974827
Drug Substance Claim :
Drug Product Claim :
Application Number : 204485
Patent Use Code : U-1857
Delist Requested :
Patent Use Description : TO INCREASE BLOOD PRES...
Patent Expiration Date : 2035-01-30

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Patent Expiration Date : 2035-01-30
US Patent Number : 9919026
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 204485
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2035-01-30

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Patent Expiration Date : 2035-01-30
US Patent Number : 9925234
Drug Substance Claim :
Drug Product Claim :
Application Number : 204485
Patent Use Code : U-1857
Delist Requested :
Patent Use Description : TO INCREASE BLOOD PRES...
Patent Expiration Date : 2035-01-30

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Patent Expiration Date : 2035-01-30
US Patent Number : 10010575
Drug Substance Claim :
Drug Product Claim :
Application Number : 204485
Patent Use Code : U-1857
Delist Requested :
Patent Use Description : TO INCREASE BLOOD PRES...
Patent Expiration Date : 2035-01-30

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Patent Expiration Date : 2035-01-30
US Patent Number : 9925233
Drug Substance Claim :
Drug Product Claim :
Application Number : 204485
Patent Use Code : U-1857
Delist Requested :
Patent Use Description : TO INCREASE BLOOD PRES...
Patent Expiration Date : 2035-01-30

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Patent Expiration Date : 2035-01-30
US Patent Number : 9968649
Drug Substance Claim :
Drug Product Claim :
Application Number : 204485
Patent Use Code : U-1857
Delist Requested :
Patent Use Description : TO INCREASE BLOOD PRES...
Patent Expiration Date : 2035-01-30

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Patent Expiration Date : 2035-01-30
US Patent Number : 9919026
Drug Substance Claim :
Drug Product Claim : Y
Application Number : 204485
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2035-01-30

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Patent Expiration Date : 2035-01-30
US Patent Number : 9375478
Drug Substance Claim :
Drug Product Claim :
Application Number : 204485
Patent Use Code : U-1857
Delist Requested :
Patent Use Description : TO INCREASE BLOOD PRES...
Patent Expiration Date : 2035-01-30

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?