
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
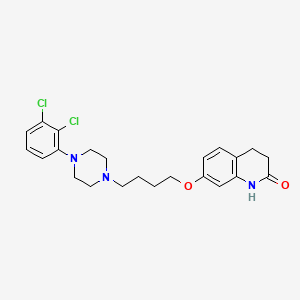

1. 7-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl)butyloxy)-3,4-dihydro-2(1h)-quinolinone
2. Abilify
3. Aripiprazol
4. Opc 14597
5. Opc-14597
1. 129722-12-9
2. Abilify
3. Abilitat
4. Opc-14597
5. Abilify Discmelt
6. Opc 14597
7. Opc 31
8. Aripiprex
9. Abilify Maintena
10. Abilify Mycite
11. Opc-31
12. 7-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1h-quinolin-2-one
13. 7-(4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy)-3,4-dihydroquinolin-2(1h)-one
14. 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydrocarbostyril
15. Nsc-759266
16. Chembl1112
17. 7-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl)butoxy)-3,4-dihydrocarbostyril
18. 7-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl)butoxy)-3,4-dihydro-2(1h)-quinolinone
19. 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydro-2(1h)-quinolinone
20. 7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}-3,4-dihydroquinolin-2(1h)-one
21. Aripiprazol
22. Discmelt
23. Chebi:31236
24. 82vfr53i78
25. 129722-12-9 (free Base)
26. Mfcd00892072
27. 2(1h)-quinolinone, 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]-3,4-dihydro-
28. 7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}-1,2,3,4-tetrahydroquinolin-2-one
29. Ncgc00159510-02
30. Aripiprazole [usan]
31. 2(1h)-quinolinone, 7-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl)butoxy)-3,4-dihydro-
32. Dsstox_cid_26083
33. Dsstox_rid_81324
34. Dsstox_gsid_46083
35. Aripiprazolum
36. 7-[4-[4-[2,3-bis(chloranyl)phenyl]piperazin-1-yl]butoxy]-3,4-dihydro-1h-quinolin-2-one
37. 7-{4-[4-(2,3-dichloro-phenyl)-piperazin-1-yl]-butoxy}-3,4-dihydro-1h-quinol In-2-one
38. Smr000466383
39. Abilify (tn)
40. Abilify Maintena Kit
41. Cas-129722-12-9
42. Hsdb 7320
43. Sr-01000759353
44. Unii-82vfr53i78
45. Abilify Digital
46. 7-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl)butyloxy)-3,4-dihydro-2(1h)-quinolinone
47. Aripiprazole [usan:inn:ban]
48. 7-(4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy)-3,4-dihydrocarbostyril
49. 7-{4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy}-3,4-dihydrocarbostyril
50. Abilify Od
51. 7-(4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy)-3,4-dihydroquinolin-2(1h)-one
52. Aripiprazole Depot
53. 9sc
54. Aripiprazole Solution
55. Aripiprazole- Bio-x
56. Bms-337039
57. Ks-1030
58. Aripiprazole (abilify)
59. Cpd000466383
60. Aripiprazole-d8 Solution
61. Abilify Mycite Kit
62. Aripiprazole [mi]
63. Aripiprazole [inn]
64. Aripiprazole [jan]
65. Gtpl34
66. Aripiprazole [hsdb]
67. Aripiprazole-d8(butyl-d8)
68. Schembl8255
69. Aripiprazole [vandf]
70. Aripiprazole [mart.]
71. Mls000759517
72. Mls001165779
73. Mls001195621
74. Mls001424078
75. Mls006011892
76. Aripiprazole [usp-rs]
77. Aripiprazole [who-dd]
78. Aripiprazole (jan/usp/inn)
79. Schembl5335696
80. Aripiprazole [ema Epar]
81. Aristada (aripiprazole Lauroxil)
82. Dtxsid3046083
83. Schembl12961254
84. Aripiprazole [orange Book]
85. Hms2051i18
86. Hms2089m20
87. Hms2093f22
88. Hms2230m18
89. Hms3373d04
90. Hms3393i18
91. Hms3657k13
92. Hms3715j07
93. Hms3744a13
94. Hms3884e18
95. Pharmakon1600-01505851
96. Aripiprazole [ep Monograph]
97. Act03221
98. Bcp04902
99. Zinc1851149
100. Aripiprazole [usp Monograph]
101. Tox21_111728
102. Bbl029082
103. Bdbm50130293
104. Dl-178
105. Nsc759266
106. S1975
107. Stk625160
108. Akos005558247
109. Tox21_111728_1
110. Ab07660
111. Ac-1554
112. Bcp9000317
113. Ccg-100891
114. Cs-0766
115. Db01238
116. Nc00141
117. Nsc 759266
118. Aripiprazole 1.0 Mg/ml In Acetonitrile
119. Ncgc00159510-03
120. Ncgc00159510-04
121. Ncgc00159510-05
122. Ba164218
123. Hy-14546
124. Sy053146
125. Sbi-0206870.p001
126. Am20090766
127. Ft-0600352
128. Ft-0662278
129. Ft-0662279
130. Sw197521-3
131. A19454
132. D01164
133. Ab00639935-09
134. Ab00639935_10
135. Ab00639935_11
136. 722a129
137. L001339
138. Q411188
139. J-005707
140. Sr-01000759353-4
141. Sr-01000759353-6
142. Brd-k70358946-001-06-6
143. Z1541632800
144. 7-[4-(4-(2,3-dichlorophenyl)piperazin-1-yl)butoxy]-3,4-dihydro-2(1h)-quinolinone
145. 7-[4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy]3,4-dihydro-2(1h)-quinolinone
146. 7-[4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-quinolin-2(1h)-one
147. 7-{4-[-4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy}-3,4-dihydrocarbostyril
148. 7-{4-[4-(2,3-dichloro-phenyl)-piperazin-1-yl]-butoxy}-3,4-dihydro-1h-quinolin-2-one
149. 7-{4-[4-(2,3-dichlorophenyl)-1-piperazinyl]butoxy}-3, 4-dihydrocarbostyril
150. 7-{4-[4-(2,3-dichlorophenyl)piperazino]butoxy}-3,4-dihydro-2(1h)-quinolinone
151. Aripiprazole; 7-(4-(4-(2,3-dichlorophenyl)-1-piperazinyl)butoxy)-3,4-dihydrocarbostyril
152. 1026778-41-5
153. 24-29-3
154. Aripiprazole Solution, 1.0 Mg/ml (50:50 Methanol/water With 1% 1n Hcl), Ampule Of 1 Ml, Certified Reference Material
155. Aripiprazole Solution, 1.0 Mg/ml In Acetonitrile, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 448.4 g/mol |
|---|---|
| Molecular Formula | C23H27Cl2N3O2 |
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 447.1480325 g/mol |
| Monoisotopic Mass | 447.1480325 g/mol |
| Topological Polar Surface Area | 44.8 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 559 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Abilify |
| PubMed Health | Aripiprazole (Injection) |
| Drug Classes | Antipsychotic |
| Drug Label | Aripiprazole is a psychotropic drug that is available as ABILIFY (aripiprazole) Tablets, ABILIFY DISCMELT (aripiprazole) Orally Disintegrating Tablets, ABILIFY (aripiprazole) Oral Solution, and ABILIFY (aripiprazole) Injection, a solution for... |
| Active Ingredient | Aripiprazole |
| Dosage Form | Solution; Injectable; Tablet, orally disintegrating; Tablet |
| Route | Intramuscular; Oral |
| Strength | 30mg; 10mg; 5mg; 9.75mg/1.3ml (7.5mg/ml); 15mg; 1mg/ml; 20mg; 2mg |
| Market Status | Prescription |
| Company | Otsuka |
| 2 of 4 | |
|---|---|
| Drug Name | Aripiprazole |
| PubMed Health | Aripiprazole |
| Drug Classes | Antipsychotic |
| Drug Label | Aripiprazole is a psychotropic drug that is available as ABILIFY (aripiprazole) Tablets, ABILIFY DISCMELT (aripiprazole) Orally Disintegrating Tablets, ABILIFY (aripiprazole) Oral Solution, and ABILIFY (aripiprazole) Injection, a solution for... |
| Active Ingredient | Aripiprazole |
| Dosage Form | Tablet, orally disintegrating; Tablet |
| Route | orally disintegrating; oral |
| Strength | 5mg; 2mg; 30mg; 10mg; 15mg; 20mg |
| Market Status | Tentative Approval |
| Company | Apotex; Alembic Pharms; Torrent Pharms; Zydus Pharms Usa; Barr Labs; Sun Pharma Global |
| 3 of 4 | |
|---|---|
| Drug Name | Abilify |
| PubMed Health | Aripiprazole (Injection) |
| Drug Classes | Antipsychotic |
| Drug Label | Aripiprazole is a psychotropic drug that is available as ABILIFY (aripiprazole) Tablets, ABILIFY DISCMELT (aripiprazole) Orally Disintegrating Tablets, ABILIFY (aripiprazole) Oral Solution, and ABILIFY (aripiprazole) Injection, a solution for... |
| Active Ingredient | Aripiprazole |
| Dosage Form | Solution; Injectable; Tablet, orally disintegrating; Tablet |
| Route | Intramuscular; Oral |
| Strength | 30mg; 10mg; 5mg; 9.75mg/1.3ml (7.5mg/ml); 15mg; 1mg/ml; 20mg; 2mg |
| Market Status | Prescription |
| Company | Otsuka |
| 4 of 4 | |
|---|---|
| Drug Name | Aripiprazole |
| PubMed Health | Aripiprazole |
| Drug Classes | Antipsychotic |
| Drug Label | Aripiprazole is a psychotropic drug that is available as ABILIFY (aripiprazole) Tablets, ABILIFY DISCMELT (aripiprazole) Orally Disintegrating Tablets, ABILIFY (aripiprazole) Oral Solution, and ABILIFY (aripiprazole) Injection, a solution for... |
| Active Ingredient | Aripiprazole |
| Dosage Form | Tablet, orally disintegrating; Tablet |
| Route | orally disintegrating; oral |
| Strength | 5mg; 2mg; 30mg; 10mg; 15mg; 20mg |
| Market Status | Tentative Approval |
| Company | Apotex; Alembic Pharms; Torrent Pharms; Zydus Pharms Usa; Barr Labs; Sun Pharma Global |
Antipsychotic Agents
National Library of Medicine's Medical Subject Headings. Aripiprazole. Online file (MeSH, 2014). Available from, as of December 18, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Aripiprazole is used IM for the acute management of agitation associated with schizophrenia or bipolar disorder, mixed or manic, in adults for whom treatment with aripiprazole is appropriate and who require an IM antipsychotic agent for rapid control of behaviors that interfere with diagnosis and care (e.g., threatening behaviors, escalating or urgently distressing behavior, self-exhausting behavior).
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2428
Aripiprazole is used orally for the acute treatment of irritability associated with autistic disorder.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2428
Aripiprazole is used orally as an adjunct to antidepressants for the acute treatment of major depressive disorder in adults.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2428
For more Therapeutic Uses (Complete) data for ARIPIPRAZOLE (6 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS. Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (eg, heart failure, sudden death) or infectious (eg, pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. ABILIFY (aripiprazole) is not approved for the treatment of patients with dementia-related psychosis. /Included in label/
NIH; DailyMed. Current Medication Information for ABILIFY- aripiprazole tablet; ABILIFY- aripiprazole solution; ABILIFY DISCMELT- aripiprazole tablet, orally disintegrating; ABILIFY- aripiprazole injection, solution (Updated: July 2014). Available from, as of March 31, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c040bd1d-45b7-49f2-93ea-aed7220b30ac
/BOXED WARNING/ WARNING: INCREASED SUICIDALTHOUGHTS AND BEHAVIORS. Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of adjunctive ABILIFY or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. ABILIFY is not approved for use in pediatric patients with depression. /Included in label/
NIH; DailyMed. Current Medication Information for ABILIFY- aripiprazole tablet; ABILIFY- aripiprazole solution; ABILIFY DISCMELT- aripiprazole tablet, orally disintegrating; ABILIFY- aripiprazole injection, solution (Updated: July 2014). Available from, as of March 31, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c040bd1d-45b7-49f2-93ea-aed7220b30ac
Contraindications: Known hypersensitivity reaction to aripiprazole or any ingredient in the formulation; such reactions have ranged from pruritus/urticaria to anaphylaxis.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2429
Safety and effectiveness in pediatric patients with bipolar mania were established in a 4-week, placebo-controlled clinical trial in 197 pediatric patients aged 10 to 17 years. The incidence of discontinuation due to adverse reactions between aripiprazole-treated and placebo-treated pediatric patients (10 to 17 years) was 7% and 2%, respectively. Commonly observed adverse reactions associated with the use of aripiprazole in pediatric patients with bipolar mania (incidence of 5% or greater and aripiprazole incidence at least twice that for placebo) somnolence, extrapyramidal disorder, fatigue, nausea, akathisia, blurred vision, salivary hypersecretion, and dizziness. Although maintenance efficacy in pediatric patients has not been systematically evaluated, maintenance efficacy can be extrapolated from adult data along with comparisons of aripiprazole pharmacokinetic parameters in adult and pediatric patients.
NIH; DailyMed. Current Medication Information for ABILIFY- aripiprazole tablet; ABILIFY- aripiprazole solution; ABILIFY DISCMELT- aripiprazole tablet, orally disintegrating; ABILIFY- aripiprazole injection, solution (Updated: July 2014). Available from, as of September 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c040bd1d-45b7-49f2-93ea-aed7220b30ac
For more Drug Warnings (Complete) data for ARIPIPRAZOLE (27 total), please visit the HSDB record page.
Aripiprazole is indicated for manic and mixed episodes associated with bipolar I disorder, irritability associated with autism spectrum disorder, treatment of schizophrenia, treatment of Tourette's disorder, and as an adjunctive treatment of major depressive disorder. An injectable formulation of aripiprazole is indicated for agitation associated with schizophrenia or bipolar mania.
FDA Label
Aripiprazole Accord is indicated for the treatment of schizophrenia in adults and in adolescents aged 15 years and older.
Aripiprazole Accord is indicated for the treatment of moderate to severe manic episodes in Bipolar I Disorder and for the prevention of a new manic episode in adults who experienced predominantly manic episodes and whose manic episodes responded to aripiprazole treatment.
Aripiprazole Accord is indicated for the treatment up to 12 weeks of moderate to severe manic episodes in Bipolar I Disorder in adolescents aged 13 years and older.
Aripiprazole Mylan Pharma is indicated for the treatment of schizophrenia in adults and in adolescents aged 15 years and older.
Aripiprazole Mylan Pharma is indicated for the treatment of moderate to severe manic episodes in Bipolar I Disorder and for the prevention of a new manic episode in adults who experienced predominantly manic episodes and whose manic episodes responded to aripiprazole treatment.
Aripiprazole Mylan Pharma is indicated for the treatment up to 12 weeks of moderate to severe manic episodes in Bipolar I Disorder in adolescents aged 13 years and older.
Abilify is indicated for the treatment of schizophrenia in adults and in adolescents aged 15 years and older.
Abilify is indicated for the treatment of moderate to severe manic episodes in Bipolar I Disorder and for the prevention of a new manic episode in adults who experienced predominantly manic episodes and whose manic episodes responded to aripiprazole treatment.
Abilify is indicated for the treatment up to 12 weeks of moderate to severe manic episodes in Bipolar I Disorder in adolescents aged 13 years and older.
Aripiprazole Zentiva is indicated for the treatment of schizophrenia in adults and in adolescents aged 15 years and older.
Aripiprazole Zentiva is indicated for the treatment of moderate to severe manic episodes in Bipolar I Disorder and for the prevention of a new manic episode in adults who experienced predominantly manic episodes and whose manic episodes responded to aripiprazole treatment.
Aripiprazole Zentiva is indicated for the treatment up to 12 weeks of moderate to severe manic episodes in Bipolar I Disorder in adolescents aged 13 years and older.
Aripiprazole Sandoz is indicated for the treatment of schizophrenia in adults and in adolescents aged 15 years and older.
Aripiprazole Sandoz is indicated for the treatment of moderate to severe manic episodes in Bipolar I Disorder and for the prevention of a new manic episode in adults who experienced predominantly manic episodes and whose manic episodes responded to aripiprazole treatment.
Aripiprazole Sandoz is indicated for the treatment up to 12 weeks of moderate to severe manic episodes in Bipolar I Disorder in adolescents aged 13 years and older.
Maintenance treatment of schizophrenia in adult patients stabilised with oral aripiprazole.
Treatment of bipolar affective disorder, Treatment of schizophrenia
Aripiprazole has high affinity for serotonin type 2 (5HT2), dopamine type 2 (D2), alpha1 and 2 adrenergic, and H1 histaminergic receptors. It also acts on a number of other receptors with lower affinity. The exact method by which aripiprazole's action on these receptors translates to a clinically relevant effect is not yet known.
Antidepressive Agents
Mood-stimulating drugs used primarily in the treatment of affective disorders and related conditions. Several MONOAMINE OXIDASE INHIBITORS are useful as antidepressants apparently as a long-term consequence of their modulation of catecholamine levels. The tricyclic compounds useful as antidepressive agents (ANTIDEPRESSIVE AGENTS, TRICYCLIC) also appear to act through brain catecholamine systems. A third group (ANTIDEPRESSIVE AGENTS, SECOND-GENERATION) is a diverse group of drugs including some that act specifically on serotonergic systems. (See all compounds classified as Antidepressive Agents.)
Dopamine Agonists
Drugs that bind to and activate dopamine receptors. (See all compounds classified as Dopamine Agonists.)
Dopamine D2 Receptor Antagonists
Compounds and drugs that bind to and inhibit or block the activation of DOPAMINE D2 RECEPTORS. (See all compounds classified as Dopamine D2 Receptor Antagonists.)
Serotonin 5-HT1 Receptor Agonists
Endogenous compounds and drugs that specifically stimulate SEROTONIN 5-HT1 RECEPTORS. Included under this heading are agonists for one or more of the specific 5-HT1 receptor subtypes. (See all compounds classified as Serotonin 5-HT1 Receptor Agonists.)
Serotonin 5-HT2 Receptor Antagonists
Drugs that bind to but do not activate SEROTONIN 5-HT2 RECEPTORS, thereby blocking the actions of SEROTONIN or SEROTONIN 5-HT2 RECEPTOR AGONISTS. Included under this heading are antagonists for one or more specific 5-HT2 receptor subtypes. (See all compounds classified as Serotonin 5-HT2 Receptor Antagonists.)
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
N05AX12
N05AX12
N05AX12
N05AX12
N05AX12
N05AX12
N05AX12
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05A - Antipsychotics
N05AX - Other antipsychotics
N05AX12 - Aripiprazole
Absorption
Aripiprazole tablets are 87% bioavailable and reach peak plasma concentrations in 3 to 5 hours. These tablets can be taken with or without food, but a high fat meal can delay the time to max concentration by 3 hours and up to 12 hours for the active metabolite.
Route of Elimination
25% of a given dose will be eliminated in urine and 55% in the feces. <1% of a dose is eliminated in the urine as unmetabolized aripiprazole and approximately 18% of a dose will be eliminated in the feces unmetabolized.
Volume of Distribution
404L or 4.9L/kg.
Clearance
0.8mL/min/kg. Other studies have reported a clearance rate of 32971042mL/hr.
Oral availability 87%. Aripiprazole is well absorbed and can be administered with or without food. Administration with a high fat meal did not affect the Cmax or AUC, but delayed Tmax by 3 hours for aripiprazole, and 12 hours for dehydro-aripiprazole.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2431
Time to peak concentration: Peak plasma concentrations: within 3 to 5 hours.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2431
The steady-state volume of distribution of aripiprazole following intravenous administration is high (404 L or 4.9 L/kg), indicating extensive extravascular distribution. At therapeutic concentrations, aripiprazole and its major metabolite are greater than 99% bound to serum proteins, primarily to albumin.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2431
There was dose-dependent D2-receptor occupancy indicating brain penetration of aripiprazole in healthy human volunteers administered 0.5 to 30 mg per day.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2431
For more Absorption, Distribution and Excretion (Complete) data for ARIPIPRAZOLE (8 total), please visit the HSDB record page.
Metabolism of aripiprazole is predominantly hepatic, mediated mostly by cytochrome P450 (CYP)3A4 and CYP2D6. These enzymes perform dehydrogenation and hydroxylation while CYP3A4 alone performs N-dealkylation. At any given time, the active metabolite dehydro-aripiprazole is approximately 40% of the drug available in plasma.
Aripiprazole is extensively metabolized in the liver principally via dehydrogenation, hydroxylations, and N-dealkylation by the cytochrome P-450 (CYP) 2D6 and 3A4 isoenzymes. The major active metabolite of aripiprazole, dehydro-aripiprazole, exhibits affinity for D2 receptors similar to that of the parent compound and represents approximately 40% of the aripiprazole area under the concentration-time curve (AUC) in plasma. Steady-state plasma concentrations of both aripiprazole and dehydro-aripiprazole are achieved within 14 days.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2433
ABILIFY activity is presumably primarily due to the parent drug, aripiprazole, and to a lesser extent, to its major metabolite, dehydro-aripiprazole, which has been shown to have affinities for D2 receptors similar to the parent drug and represents 40% of the parent drug exposure in plasma.
NIH; DailyMed. Current Medication Information for ABILIFY- aripiprazole tablet; ABILIFY- aripiprazole solution; ABILIFY DISCMELT- aripiprazole tablet, orally disintegrating; ABILIFY- aripiprazole injection, solution (Updated: July 2014). Available from, as of September 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c040bd1d-45b7-49f2-93ea-aed7220b30ac
Aripiprazole has known human metabolites that include 2,3-dichlorophenylpiperazine, 4-Hydroxyaripiprazole, 4-[(2-oxo-3,4-dihydro-1H-quinolin-7-yl)oxy]butanal, and dehydro-aripiprazole.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The half life of aripiprazole is 75 hours while the half life of the active metabolite is 94 hours. For populations that are poor CYP2D6 metabolizers, the half life of aripiprazole is 146 hours and these patients should be treated with half the normal dose. Other studies have reported a half life of 61.0319.59 hours for aripiprazole and 279299 hours for the active metabolite.
The mean elimination half-lives are about 75 hours and 94 hours for aripiprazole and dehydro-aripiprazole, respectively.
NIH; DailyMed. Current Medication Information for ABILIFY- aripiprazole tablet; ABILIFY- aripiprazole solution; ABILIFY DISCMELT- aripiprazole tablet, orally disintegrating; ABILIFY- aripiprazole injection, solution (Updated: July 2014). Available from, as of September 4, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c040bd1d-45b7-49f2-93ea-aed7220b30ac
The antipsychotic action of aripiprazole is likely due to the agonism of D2 and 5-HT1A receptors though the exact mechanism has not been defined. Some adverse effects may be due to action on other receptors. For example, orthostatic hypotension may be explained by antagonism of the adrenergic alpha1 receptors.
The exact mechanism of antipsychotic action of aripiprazole has not been fully elucidated but, like that of other atypical antipsychotic agents (e.g.,olanzipine, risperidone, ziprasidon), may involve the drug's activity at dopamine D2 and serotonin type (5-HT1A) and type 2 (5-HT2A) receptors. However, aripiprazole appears to differ from other atypical antipsychotic agents because the drug demonstrates partial agonist activity at D2 and 5-HT1A receptors and antagonist activity at 5-HT2A receptors. Antagonism at other receptors (e.g., alpha1-adrenergic receptors, histamine H1 receptors) may contribute to other therapeutic and adverse effects (e.g., orthostatic hypotension, somnolence) observed with aripiprazole.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015, p. 2432
...Aripiprazole exhibits typical antagonism at dopamine (D2) receptors in the mesolimbic pathway, as well as having unique partial agonist activity at D2 receptors in the mesocortical pathway. As exemplified by other atypical antipsychotics, it displays strong 5-HT(2a) receptor antagonism and is similar to ziprasidone in also having agonistic activity at the 5-HT(1a) receptor. Among the atypical antipsychotics, aripiprazole displays the lowest affinity for alpha(1)adrenergic (alpha(1)), histamine (H1) and muscarinic (M1) receptors. This combination of effects may be responsible for its efficacy in positive and negative symptoms of schizophrenia and in bipolar disorder. ...Other early data suggest that aripiprazole may induce reductions in plasma prolactin, as well as in plasma glucose and lipid profiles ...
PMID:12472374 Goodnick PJ, Jerry JM; Expert Opin Pharmacother 3 (12): 1773-81 (2002)
 Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
 Click Us!
Click Us! 
GDUFA
DMF Review : Reviewed
Rev. Date : 2021-11-17
Pay. Date : 2020-12-02
DMF Number : 34969
Submission : 2021-03-31
Status : Active
Type : II

NDC Package Code : 64552-4073
Start Marketing Date : 2013-02-28
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Date of Issue : 2022-12-09
Valid Till : 2025-07-02
Written Confirmation Number : WC-0156A2
Address of the Firm :
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 33204
Submission : 2018-10-30
Status : Active
Type : II

NDC Package Code : 12658-0542
Start Marketing Date : 2002-11-15
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (6kg/6kg)
Marketing Category : BULK INGREDIENT

| Available Reg Filing : ASMF |
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.

GDUFA
DMF Review : Reviewed
Rev. Date : 2017-11-16
Pay. Date : 2017-10-18
DMF Number : 30120
Submission : 2015-12-14
Status : Active
Type : II

Certificate Number : CEP 2016-021 - Rev 02
Issue Date : 2024-07-09
Type : Chemical
Substance Number : 2617
Status : Valid

Date of Issue : 2022-08-25
Valid Till : 2025-07-02
Written Confirmation Number : WC-0195
Address of the Firm :

NDC Package Code : 76072-1002
Start Marketing Date : 2016-04-01
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Hwail Pharmaceutical Co., Ltd.
Registration Date : 2018-03-16
Registration Number : 20180316-209-J-68
Manufacturer Name : ZCL Chemicals Limited
Manufacturer Address : Plot No. 3102/B GIDC, Industrial Estate Ankleshwar-393 002, Dist. Bharuch, Gujarat state, India
 Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.
Egis is a Hungarian generic pharma company with 110 years history. Our activities incorporate all areas of the pharma value chain.

Certificate Number : R1-CEP 2014-252 - Rev 02
Issue Date : 2022-07-21
Type : Chemical
Substance Number : 2617
Status : Valid

GDUFA
DMF Review : Reviewed
Rev. Date : 2021-11-18
Pay. Date : 2021-08-10
DMF Number : 22889
Submission : 2009-06-24
Status : Active
Type : II

Date of Issue : 2023-06-23
Valid Till : 2026-06-29
Written Confirmation Number : WC-0478
Address of the Firm :

NDC Package Code : 58032-2005
Start Marketing Date : 2017-12-14
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
GDUFA
DMF Review : Complete
Rev. Date : 2021-11-17
Pay. Date : 2020-12-02
DMF Number : 34969
Submission : 2021-03-31
Status : Active
Type : II
 Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
GDUFA
DMF Review : Complete
Rev. Date : 2022-08-29
Pay. Date : 2022-07-13
DMF Number : 37184
Submission : 2022-08-02
Status : Active
Type : II
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 33204
Submission : 2018-10-30
Status : Active
Type : II
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
GDUFA
DMF Review : Complete
Rev. Date : 2015-02-20
Pay. Date : 2013-12-19
DMF Number : 23314
Submission : 2009-11-27
Status : Active
Type : II
 Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
GDUFA
DMF Review : Complete
Rev. Date : 2017-11-16
Pay. Date : 2017-10-18
DMF Number : 30120
Submission : 2015-12-14
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2023-09-26
Pay. Date : 2023-08-28
DMF Number : 36451
Submission : 2021-11-06
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2021-11-18
Pay. Date : 2021-08-10
DMF Number : 22889
Submission : 2009-06-24
Status : Active
Type : II
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15651
Submission : 2001-10-05
Status : Inactive
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18774
Submission : 2005-09-13
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2015-05-15
Pay. Date : 2014-04-14
DMF Number : 18706
Submission : 2005-08-15
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
NDC Package Code : 64552-4085
Start Marketing Date : 2013-02-28
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
NDC Package Code : 64552-4073
Start Marketing Date : 2013-02-28
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
NDC Package Code : 64552-4085
Start Marketing Date : 2013-02-28
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
NDC Package Code : 12658-0577
Start Marketing Date : 2002-11-15
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (4kg/4kg)
Marketing Category : BULK INGREDIENT
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
NDC Package Code : 12658-0542
Start Marketing Date : 2002-11-15
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (6kg/6kg)
Marketing Category : BULK INGREDIENT
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
NDC Package Code : 12658-0601
Start Marketing Date : 2002-11-15
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (8kg/8kg)
Marketing Category : BULK INGREDIENT
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
NDC Package Code : 12658-0602
Start Marketing Date : 2002-11-15
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (10kg/10kg)
Marketing Category : BULK INGREDIENT
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
NDC Package Code : 12658-0576
Start Marketing Date : 2002-11-15
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (2kg/2kg)
Marketing Category : BULK INGREDIENT
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
NDC Package Code : 12658-0623
Start Marketing Date : 2002-11-15
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
NDC Package Code : 12658-0599
Start Marketing Date : 2002-11-15
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (5kg/5kg)
Marketing Category : BULK INGREDIENT
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
Interquim comes from an international group of 50 companies active in the pharma, hospital, diagnostics, fine chemicals & feed sectors.
About the Company : Interquim, founded in 1978, is now part of the Ferrer HealthTech division. Interquim specializes in the development of competitive processes for manufacturing high added-value APIs...
About the Company : Bal Pharma is a leading Indian pharmaceutical company with 30+ years of experience, specializing in prescription drugs, generic and OTC products, intravenous infusions, and bulk ac...
 Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
Polpharma is a Polish CDMO of APIs and a significant European API producer, delivering products to companies worldwide.
About the Company : Polpharma API. European CDMO partner and API manufacturer since 1951. Poland-based CDMO and API producer, delivering products to pharmaceutical companies worldwide. Aiming at the c...
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for the pharmaceutical and biotech industries. LGM is also a full service CDMO providing formulation, ...
 Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
Cohance Lifesciences, offers full range of CDMO services for small molecule APIs, intermediates, ADCs, Pellets and Formulations.
About the Company : Cohance Lifesciences is a leading CDMO and API platform, offering products and services across all phases of a molecule’s lifecycle from development to commercialzation. With our...
About the Company : Jai Radhe Sales was founded in 1999 as an out-of-the-box distribution firm specializing in the global supply of high-quality pharmaceutical ingredients. The firm provides complete ...
About the Company : HRV Global is a leading global manufacturer, seller & exporter of a wide range of APIs, advanced intermediates, pellets, food grade chemicals, food additives & food ingredients. It...
About the Company : Established in 1984, Neuland Laboratories Limited is a publicly listed company headquartered in Hyderabad, India. The company provides solutions across the entire spectrum of the p...
 Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
Octavius has been empowering lives since 1980 by providing quality products like DC granules, APIs and FDFs.
About the Company : Octavius Pharma is a global leader in Directly Compressible Granules with over 40 years of experience in Formulation development, manufacturing and commercialization. It offers a w...
About the Company : We, Hana Pharmaceutical Co., Ltd. was founded the name of Woo-Chun Pharmaceutical Company in 1958. We also changed our company name to Hana in 1996. Our aim to support people can ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Abilify Maintena (aripiprazole) is an atypical antipsychotic approved for maintaining treatment of schizophrenia in adults stabilized with aripiprazole.
Lead Product(s): Aripiprazole
Therapeutic Area: Psychiatry/Psychology Brand Name: Abilify Maintena
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable March 27, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aripiprazole
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Abilify Maintena® Approved in EU as Long-Acting Injectable for Schizophrenia
Details : Abilify Maintena (aripiprazole) is an atypical antipsychotic approved for maintaining treatment of schizophrenia in adults stabilized with aripiprazole.
Brand Name : Abilify Maintena
Molecule Type : Small molecule
Upfront Cash : Not Applicable
March 27, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Aripiprazole action includes a combination of partial agonist activity at D2 and 5-HT1A receptors and antagonist activity at 5-HT2A receptors. It is approved by USFDA for the treatment of schizophrenia and bipolar I disorder in adults.
Lead Product(s): Aripiprazole
Therapeutic Area: Psychiatry/Psychology Brand Name: Abilify-Generic
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aripiprazole
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Unichem Laboratories Limited Receives Tentative USFDA Approval for ANDA of Aripiprazole Tablets
Details : Aripiprazole action includes a combination of partial agonist activity at D2 and 5-HT1A receptors and antagonist activity at 5-HT2A receptors. It is approved by USFDA for the treatment of schizophrenia and bipolar I disorder in adults.
Brand Name : Abilify-Generic
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Abilify (aripiprazole) action includes a combination of partial agonist activity at D2 and 5-HT1A receptors and antagonist activity at 5-HT2A receptors. It is approved by USFDA for the treatment of schizophrenia and for bipolar I disorder in adults.
Lead Product(s): Aripiprazole
Therapeutic Area: Psychiatry/Psychology Brand Name: Abilify Asimtufii
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Otsuka Holdings
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable April 28, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aripiprazole
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Otsuka Holdings
Deal Size : Not Applicable
Deal Type : Not Applicable
FDA Approves Otsuka and Lundbeck’s ABILIFY ASIMTUFII® (aripiprazole), the First Once-Every-Two-...
Details : Abilify (aripiprazole) action includes a combination of partial agonist activity at D2 and 5-HT1A receptors and antagonist activity at 5-HT2A receptors. It is approved by USFDA for the treatment of schizophrenia and for bipolar I disorder in adults.
Brand Name : Abilify Asimtufii
Molecule Type : Small molecule
Upfront Cash : Not Applicable
April 28, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Abilify Maintena (aripiprazole) is a long-acting injectable provided in a single-chamber-type, ready-to-use pre-filled syringe, intended for dosing every two months via IM injection in the gluteal muscle in the same patient as indicated for once-monthly ABILIFY MAINTENA.
Lead Product(s): Aripiprazole
Therapeutic Area: Psychiatry/Psychology Brand Name: Abilify Maintena
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: H. Lundbeck AS
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable April 05, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aripiprazole
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : H. Lundbeck AS
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Abilify Maintena (aripiprazole) is a long-acting injectable provided in a single-chamber-type, ready-to-use pre-filled syringe, intended for dosing every two months via IM injection in the gluteal muscle in the same patient as indicated for once-monthly ...
Brand Name : Abilify Maintena
Molecule Type : Small molecule
Upfront Cash : Not Applicable
April 05, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
If approved, Abilify (aripiprazole) 2-month ready-to-use, long-acting injectable (LAI) would be the first 2-month, LAI antipsychotic indicated for both treatment of schizophrenia and the maintenance treatment of bipolar I disorder in U.S.
Lead Product(s): Aripiprazole
Therapeutic Area: Psychiatry/Psychology Brand Name: Abilify Asimtufii
Study Phase: Phase IIIProduct Type: Small molecule
Sponsor: H. Lundbeck AS
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 13, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aripiprazole
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : H. Lundbeck AS
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : If approved, Abilify (aripiprazole) 2-month ready-to-use, long-acting injectable (LAI) would be the first 2-month, LAI antipsychotic indicated for both treatment of schizophrenia and the maintenance treatment of bipolar I disorder in U.S.
Brand Name : Abilify Asimtufii
Molecule Type : Small molecule
Upfront Cash : Not Applicable
September 13, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Aripiprazole is dopamine D2 partial agonist with weak 5-HT1a partial agonism and 5-HT2A receptor antagonism. It is available as a daily oral tablet, orally disintegrating tablet, and oral solution (Abilify®), or a once monthly LAI formulation (Abilify Maintena®).
Lead Product(s): Aripiprazole
Therapeutic Area: Psychiatry/Psychology Brand Name: Abilify Maintena
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 17, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aripiprazole
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Aripiprazole is dopamine D2 partial agonist with weak 5-HT1a partial agonism and 5-HT2A receptor antagonism. It is available as a daily oral tablet, orally disintegrating tablet, and oral solution (Abilify®), or a once monthly LAI formulation (Abilify M...
Brand Name : Abilify Maintena
Molecule Type : Small molecule
Upfront Cash : Not Applicable
June 17, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Otsuka Pharmaceutical created ABILFY MAINTENA, an atypical antipsychotic, which first received regulatory approval in 2013 in the U.S. and E.U. for the treatment of adult schizophrenia.
Lead Product(s): Aripiprazole
Therapeutic Area: Psychiatry/Psychology Brand Name: Abilify Maintena
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable September 26, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aripiprazole
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Otsuka Pharmaceutical created ABILFY MAINTENA, an atypical antipsychotic, which first received regulatory approval in 2013 in the U.S. and E.U. for the treatment of adult schizophrenia.
Brand Name : Abilify Maintena
Molecule Type : Small molecule
Upfront Cash : Not Applicable
September 26, 2020

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : ARIPIPRAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 2MG
Approval Date : 2017-01-09
Application Number : 91279
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : ARIPIPRAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 5MG
Approval Date : 2017-01-09
Application Number : 91279
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : ARIPIPRAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 10MG
Approval Date : 2017-01-09
Application Number : 91279
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : ARIPIPRAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 15MG
Approval Date : 2017-01-09
Application Number : 91279
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : ARIPIPRAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 20MG
Approval Date : 2017-01-09
Application Number : 91279
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code :
Brand Name : ARIPIPRAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 30MG
Approval Date : 2017-01-09
Application Number : 91279
RX/OTC/DISCN : DISCN
RLD : No
TE Code :
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : ARIPIPRAZOLE
Dosage Form : TABLET, ORALLY DISINTEGRATING; ORAL
Dosage Strength : 15MG
Approval Date :
Application Number : 78612
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : ARIPIPRAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 2MG
Approval Date : 2024-03-18
Application Number : 205589
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : ABILIFY MAINTENA KIT
Dosage Form : FOR SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR
Dosage Strength : 400MG
Approval Date : 2014-09-29
Application Number : 202971
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : No
TE Code : AB
Brand Name : ARIPIPRAZOLE
Dosage Form : TABLET;ORAL
Dosage Strength : 10MG
Approval Date : 2016-09-29
Application Number : 206383
RX/OTC/DISCN : RX
RLD : No
TE Code : AB

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?