
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
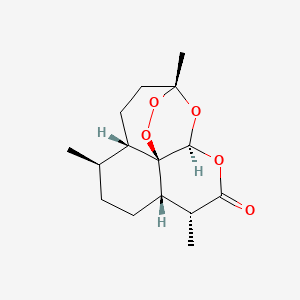

1. (3r,5as,6r,8as,9r,12s,12ar)-3,6,9-trimethyloctahydro-3,12-epoxypyrano(4,3-j)-1,2-benzodioxepin-10(3h)-one
2. Arteannuin
3. Artemisinine
4. Artemisininum
5. Huanghuahaosu
6. Qing Hao Su
7. Qinghaosu
8. Quing Hau Sau
9. Quinghaosu
1. Qinghaosu
2. 63968-64-9
3. Huanghuahaosu
4. Artemisinine
5. Artemisine
6. (+)-artemisinin
7. Artemisinina
8. Artemisininum
9. Artemisinin [inn]
10. Qing Hau Sau
11. Nsc 369397
12. Chebi:223316
13. Octahydro-3,6,9-trimethyl-3,12-epoxy-12h-pyrano(4,3-j)-1,2-benzodioxepin-10(3h)-one
14. 9rmu91n5k2
15. Qinghosu
16. Quing Hau Sau
17. Astemisinin
18. (1r,4s,5r,8s,9r,12s,13r)-1,5,9-trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.04,13.08,13]hexadecan-10-one
19. (3r,5as,6r,8as,9r,12s,12ar)-3,6,9-trimethyloctahydro-3,12-epoxypyrano[4,3-j][1,2]benzodioxepin-10(3h)-one
20. (3r,5as,6r,8as,9r,12s,12ar)-octahydro-3,6,9-trimethyl-3,12-epoxy-12h-pyrano(4,3-j)-1,2-benzodioxepin-10(3h)-one
21. Qing Hau Su
22. Qinghaosu [chinese]
23. Artemisinine [french]
24. Artemisininum [latin]
25. Artemisinina [spanish]
26. (+)-arteannuin
27. Qing Hau Sau [chinese]
28. Mfcd00081057
29. Artemisia Annua L., Extract
30. (1r,4s,5r,8s,9r,12s,13r)-1,5,9-trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.0^{4,13}.0^{8,13}]hexadecan-10-one
31. (3r,5as,6r,8as,9r,12s,12ar)-3,6,9-trimethyloctahydro-3,12-epoxypyrano(4,3-j)(1,2)benzodioxepin-10(3h)-one
32. (3r,5as,6r,8as,9r,12s,12ar)-octahydro-3,6,9-trimethyl-3,12-epoxy-12h-pyrano[4,3-j]-1,2-benzodioxepin-10(3h)-one
33. 1,5,9-trimethyl-(1r,4s,5r,9r,12s,13r)-11,14,15,16-tetraoxatetracyclo[10.3.1.04,13.08,13]hexadecan-10-one
34. Smr000578089
35. Sr-01000802560
36. Gnf-pf-5341
37. Artemsinin
38. Brn 4194670
39. Unii-9rmu91n5k2
40. Artemesinin
41. Artesin
42. Nsc-369397
43. Qinghaosu,artemisinine
44. Spectrum_001351
45. Artemisinin [mi]
46. Prestwick0_000498
47. Prestwick1_000498
48. Prestwick2_000498
49. Prestwick3_000498
50. Spectrum2_001512
51. Spectrum3_001549
52. Spectrum4_000721
53. Spectrum5_001098
54. Artemisinin [inci]
55. Ec 700-290-5
56. Artemisinin [mart.]
57. Schembl60304
58. Artemisinin [usp-rs]
59. Artemisinin [who-dd]
60. Artemisinin [who-ip]
61. Bspbio_000395
62. Bspbio_002998
63. Kbiogr_000982
64. Kbioss_001831
65. Mls001097650
66. Mls001304036
67. Mls002153846
68. Divk1c_000656
69. Spbio_001583
70. Spbio_002316
71. Bpbio1_000435
72. Chembl269671
73. Gtpl9954
74. Dtxsid2040652
75. Kbio1_000656
76. Kbio2_001831
77. Kbio2_004399
78. Kbio2_006967
79. Kbio3_002498
80. Ninds_000656
81. Hms2090i17
82. Hms2096d17
83. Hms2267n09
84. Hms3269l13
85. Hms3413p13
86. Hms3677p13
87. Hms3713d17
88. Pharmakon1600-01503042
89. 3,12-epoxy-12h-pyrano[4,3-j]-1,2-benzodioxepin-10(3h)-one, Octahydro-3,6,9-trimethyl-, (3r,5as,6r,8as,9r,12s,12ar)-
90. Bcp02096
91. Hy-b0094
92. Zinc8143788
93. Artemisininum [who-ip Latin]
94. Bdbm50088447
95. Nsc758216
96. Akos024457231
97. Ccg-220498
98. Cs-1794
99. Db13132
100. Lmpr0103190003
101. Nsc-758216
102. Idi1_000656
103. (3r,5as,6r,8as,9r,12s,12ar)-3,6,9-trimethyloctahydro-12h-3,12-epoxy[1,2]dioxepino[4,3-i]isochromen-10(3h)-one
104. 3,12-epoxy-12h-pyranol(4,3-j)-1,2-benzodioxepin-10(3h)-one, Octahydro-3,6,9-trimethyl-, (3-alpha,5a-beta,6-beta,8a-beta,9-alpha,12-beta,12ar*)-(+)-
105. As-12692
106. S1282
107. 968a649
108. Q426921
109. Sr-01000802560-2
110. Sr-01000802560-3
111. Sr-01000802560-4
112. Brd-k13112821-001-08-3
113. Brd-k13112821-001-17-4
114. 2ab44f63-5d0f-424a-aa3f-24062f9c1ced
115. (3r,5as,6r,8as,9r,12s,12ar)-3,6,9-trimethyloctahydro-3,12-epoxypyrano(4,3-j )-1,2-benzodioxepin-10(3h)-one
116. (3r,5as,6r,8as,9r,12s,12ar)-3,6,9-trimethyloctahydro-3h-3,12-epoxy[1,2]dioxepino[4,3-i]isochromen-10(12h)-one
| Molecular Weight | 282.33 g/mol |
|---|---|
| Molecular Formula | C15H22O5 |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 0 |
| Exact Mass | 282.14672380 g/mol |
| Monoisotopic Mass | 282.14672380 g/mol |
| Topological Polar Surface Area | 54 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 452 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01B - Antimalarials
P01BE - Artemisinin and derivatives, plain
P01BE01 - Artemisinin

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Artesun (artesunate) is rapidly metabolized into an active metabolite DHA which contains an endoperoxide bridge that is activated by heme iron leading to oxidative stress, inhibition of protein and nucleic acid synthesis, which decreases parasite growth and survival.
Lead Product(s): Artemisinin
Therapeutic Area: Infections and Infectious Diseases Brand Name: Artesun
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 26, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Artemisinin
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : Artesun (artesunate) is rapidly metabolized into an active metabolite DHA which contains an endoperoxide bridge that is activated by heme iron leading to oxidative stress, inhibition of protein and nucleic acid synthesis, which decreases parasite growth ...
Product Name : Artesun
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
April 26, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
OT-101 (trabedersen) has completed seven clinical trials including one phase 2 trial in COVID and two phase 2 trials in brain cancer and against pancreatic cancer. It has pediatric designation for a rare form of pediatric brain cancer known as DIPG.
Lead Product(s): Trabedersen,Artemisinin
Therapeutic Area: Infections and Infectious Diseases Brand Name: OT-101
Study Phase: Phase IIProduct Type: Oligonucleotide
Sponsor: BARDA
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Funding December 10, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Trabedersen,Artemisinin
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : BARDA
Deal Size : Undisclosed
Deal Type : Funding
Oncotelic Awarded BARDA Funding for Development of OT-101 for long COVID
Details : OT-101 (trabedersen) has completed seven clinical trials including one phase 2 trial in COVID and two phase 2 trials in brain cancer and against pancreatic cancer. It has pediatric designation for a rare form of pediatric brain cancer known as DIPG.
Product Name : OT-101
Product Type : Oligonucleotide
Upfront Cash : Undisclosed
December 10, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The study includes 150 adult patients with a confirmed SARS-CoV-2 infection, at least 4 weeks of long-COVID symptoms, and a Post Covid Functional Score (PCFS) between one and four. ArtemiC Support is administered orally, in a dosage of 5 drops three times daily for 6 weeks.
Lead Product(s): Artemisinin,Curcumin,Frankincense
Therapeutic Area: Infections and Infectious Diseases Brand Name: ArtemiC
Study Phase: Phase IIProduct Type: Other Small Molecule
Sponsor: MGC Pharmaceuticals
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 21, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Artemisinin,Curcumin,Frankincense
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : MGC Pharmaceuticals
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : The study includes 150 adult patients with a confirmed SARS-CoV-2 infection, at least 4 weeks of long-COVID symptoms, and a Post Covid Functional Score (PCFS) between one and four. ArtemiC Support is administered orally, in a dosage of 5 drops three time...
Product Name : ArtemiC
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
December 21, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
PulmoHeal/ ArtiVeda has proven active against COVID-19 in ARTI-19 clinical trial (A Prospective, Randomized, Multi-center, Open label, Interventional Study to Evaluate the Safety and Efficacy of Artemisinin 500 mg capsule in Treatment of Adult Subjects with COVID-19).
Lead Product(s): Artemisinin
Therapeutic Area: Infections and Infectious Diseases Brand Name: PulmoHeal
Study Phase: Phase IVProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 24, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Artemisinin
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase IV
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Pulmoheal™/ Artiveda™ Is Clinically Active Against Mild and Moderate Covid-19
Details : PulmoHeal/ ArtiVeda has proven active against COVID-19 in ARTI-19 clinical trial (A Prospective, Randomized, Multi-center, Open label, Interventional Study to Evaluate the Safety and Efficacy of Artemisinin 500 mg capsule in Treatment of Adult Subjects w...
Product Name : PulmoHeal
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
August 24, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Mateon has completed the enrollment of its sentinel Part 1 and Part 2 COVID-19 patients which allows for the continuing expansion to the targeted 18 patients in part 1 and 18 patients in part 2. OT-101 is an antisense against the host TGF-β protein.
Lead Product(s): Trabedersen,Artemisinin
Therapeutic Area: Infections and Infectious Diseases Brand Name: OT-101
Study Phase: Phase IIProduct Type: Oligonucleotide
Sponsor: Oncotelic Therapeutics
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 03, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Trabedersen,Artemisinin
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Oncotelic Therapeutics
Deal Size : Inapplicable
Deal Type : Inapplicable
Mateon’s Update on C001 - Global Study for Ot-101 Against Covid-19
Details : Mateon has completed the enrollment of its sentinel Part 1 and Part 2 COVID-19 patients which allows for the continuing expansion to the targeted 18 patients in part 1 and 18 patients in part 2. OT-101 is an antisense against the host TGF-β protein.
Product Name : OT-101
Product Type : Oligonucleotide
Upfront Cash : Inapplicable
August 03, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Manus Bio has received a third funding award from the Bill & Melinda Gates Foundation to accelerate the development of a scalable and cost-effective production method for artemisinin, a key therapeutic for treating malaria.
Lead Product(s): Artemisinin
Therapeutic Area: Infections and Infectious Diseases Brand Name: Undisclosed
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Bill & Melinda Gates Foundation
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Funding January 04, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Artemisinin
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Bill & Melinda Gates Foundation
Deal Size : Undisclosed
Deal Type : Funding
Manus Bio Awarded Additional Funding To Support Its Artemisinin Development Program
Details : Manus Bio has received a third funding award from the Bill & Melinda Gates Foundation to accelerate the development of a scalable and cost-effective production method for artemisinin, a key therapeutic for treating malaria.
Product Name : Undisclosed
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
January 04, 2021

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The Malta facility will create a European manufacturing hub for ArtemiCTM with the ability to scale production to meet expected growing demand following positive clinical trial results.
Lead Product(s): Artemisinin,Boswellia Serata,Curcumin
Therapeutic Area: Infections and Infectious Diseases Brand Name: ArtemiC
Study Phase: Phase IIProduct Type: Other Small Molecule
Sponsor: Malta Enerprises
Deal Size: $3.7 million Upfront Cash: $3.7 million
Deal Type: Funding December 22, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Artemisinin,Boswellia Serata,Curcumin
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Malta Enerprises
Deal Size : $3.7 million
Deal Type : Funding
MGC Pharmaceuticals Receives Cash Grant to Help Establish ArtemiCTM Productioninn Malta
Details : The Malta facility will create a European manufacturing hub for ArtemiCTM with the ability to scale production to meet expected growing demand following positive clinical trial results.
Product Name : ArtemiC
Product Type : Other Small Molecule
Upfront Cash : $3.7 million
December 22, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The trial is assessing the safety and efficacy of the natural anti-inflammatory formulation ArtemiCTM, a natural supplement formula based on Artemisinin and Curcumin (along with supporting ingredients Vitamin C and Boswellia serrata) on COVID-19 Patients.
Lead Product(s): Artemisinin,Curcumin,Frankincense
Therapeutic Area: Infections and Infectious Diseases Brand Name: ArtemiC
Study Phase: Phase IIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable September 28, 2020

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Artemisinin,Curcumin,Frankincense
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
MGC Pharmaceuticals Expands ArtemiC™ Phase II Clinical Trial on COVID-19 Patients in Israel and ...
Details : The trial is assessing the safety and efficacy of the natural anti-inflammatory formulation ArtemiCTM, a natural supplement formula based on Artemisinin and Curcumin (along with supporting ingredients Vitamin C and Boswellia serrata) on COVID-19 Patients...
Product Name : ArtemiC
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
September 28, 2020

Details:
ARTI19 is designed to rapidly establish the clinical efficacy of Artemisinin in mild and moderate COVID-19 patients. In-vitro, the medical grade version of the supplement has proven potency and safety similar to Remdesivir with an EC50 = 0.45 ug/ml and Safety Index = 140.
Lead Product(s): Artemisinin
Therapeutic Area: Infections and Infectious Diseases Brand Name: Undisclosed
Study Phase: PreclinicalProduct Type: Other Small Molecule
Sponsor: Mateon Therapeutics
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership July 20, 2020

Lead Product(s) : Artemisinin
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Preclinical
Partner/Sponsor/Collaborator : Mateon Therapeutics
Deal Size : Undisclosed
Deal Type : Partnership
Details : ARTI19 is designed to rapidly establish the clinical efficacy of Artemisinin in mild and moderate COVID-19 patients. In-vitro, the medical grade version of the supplement has proven potency and safety similar to Remdesivir with an EC50 = 0.45 ug/ml and S...
Product Name : Undisclosed
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
July 20, 2020

Details:
Mateon Therapeutics announced that it will fund observational studies for Artemisinin, an herbal supplement, that demonstrated potent in vitro activity against SARS-CoV-2, the COVID-19 virus.
Lead Product(s): Artemisinin
Therapeutic Area: Infections and Infectious Diseases Brand Name: Undisclosed
Study Phase: PreclinicalProduct Type: Other Small Molecule
Sponsor: Mateon Therapeutics
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Funding July 13, 2020

Lead Product(s) : Artemisinin
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Preclinical
Partner/Sponsor/Collaborator : Mateon Therapeutics
Deal Size : Undisclosed
Deal Type : Funding
Mateon Therapeutics to Fund Observational Studies of Artemisinin in Developing Countries
Details : Mateon Therapeutics announced that it will fund observational studies for Artemisinin, an herbal supplement, that demonstrated potent in vitro activity against SARS-CoV-2, the COVID-19 virus.
Product Name : Undisclosed
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
July 13, 2020

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
97
PharmaCompass offers a list of Artemisinin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Artemisinin manufacturer or Artemisinin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Artemisinin manufacturer or Artemisinin supplier.
PharmaCompass also assists you with knowing the Artemisinin API Price utilized in the formulation of products. Artemisinin API Price is not always fixed or binding as the Artemisinin Price is obtained through a variety of data sources. The Artemisinin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Artemisinin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Artemisinin, including repackagers and relabelers. The FDA regulates Artemisinin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Artemisinin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Artemisinin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Artemisinin supplier is an individual or a company that provides Artemisinin active pharmaceutical ingredient (API) or Artemisinin finished formulations upon request. The Artemisinin suppliers may include Artemisinin API manufacturers, exporters, distributors and traders.
click here to find a list of Artemisinin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Artemisinin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Artemisinin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Artemisinin GMP manufacturer or Artemisinin GMP API supplier for your needs.
A Artemisinin CoA (Certificate of Analysis) is a formal document that attests to Artemisinin's compliance with Artemisinin specifications and serves as a tool for batch-level quality control.
Artemisinin CoA mostly includes findings from lab analyses of a specific batch. For each Artemisinin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Artemisinin may be tested according to a variety of international standards, such as European Pharmacopoeia (Artemisinin EP), Artemisinin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Artemisinin USP).
