
Synopsis
Synopsis
0
USDMF
0
JDMF
0
KDMF
0
VMF
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
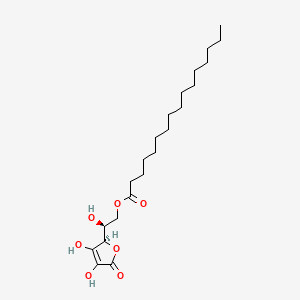

1. 6-o-palmitoylascorbate
2. 6-o-palmitoylascorbic Acid
3. Asc-6-o-palmitate
4. Asc6plm
5. Ascorbate 6-palmitate
6. Ascorbic Acid-6-o-palmitate
7. Vitamin C-palmitate
1. 137-66-6
2. L-ascorbyl 6-palmitate
3. 6-o-palmitoyl-l-ascorbic Acid
4. 6-o-palmitoylascorbic Acid
5. L-ascorbic Acid 6-palmitate
6. L-ascorbic Acid, 6-hexadecanoate
7. L-ascorbyl Palmitate
8. Ascorbyl Monopalmitate
9. Ascorbic Palmitate
10. (s)-2-((r)-3,4-dihydroxy-5-oxo-2,5-dihydrofuran-2-yl)-2-hydroxyethyl Palmitate
11. Cetyl Ascorbate
12. L-ascorbic Acid 6-hexadecanoate
13. Ins No.304
14. Ascorbyl Palmitate [nf]
15. Ins-304
16. Qn83us2b0n
17. Ascorboyl Palmitate
18. Ascorbylpalmitic Acid
19. Mfcd00005377
20. Ascorbyl 6-palmitate
21. Nsc-402451
22. E304
23. Ascorbyl Palmitate (nf)
24. E-304
25. Ascorbic Acid 6-palmitate
26. Dsstox_cid_21611
27. Dsstox_rid_79785
28. 6-palmitoyl-l-ascorbic Acid
29. Dsstox_gsid_41611
30. L-ascorbyl 6-pal
31. Grindox 562
32. Nsc 402451
33. [(2s)-2-[(2r)-3,4-dihydroxy-5-oxo-2h-furan-2-yl]-2-hydroxyethyl] Hexadecanoate
34. Cas-137-66-6
35. 6-o-palmitoyl Ascorbate
36. Ascorbyl Palmitate (van)
37. Unii-qn83us2b0n
38. Ascorbyl 6-hexadecanoate, L-
39. Ccris 3930
40. Hsdb 418
41. Ascorbic Acid Palmitate (ester)
42. Ncgc00161605-01
43. Einecs 205-305-4
44. E 304
45. Brn 0096552
46. Schembl15363
47. 5-18-05-00031 (beilstein Handbook Reference)
48. Chembl220190
49. Ascorbyl Palmitate [ii]
50. Ascorbyl Palmitate [fcc]
51. Dtxsid3041611
52. Schembl17817668
53. Ascorbyl Palmitate [hsdb]
54. Ascorbyl Palmitate [inci]
55. Chebi:140768
56. Ascorbyl Palmitate [vandf]
57. Ascorbyl Palmitate [mart.]
58. Ascorbyl Palmitate [usp-rs]
59. Ascorbyl Palmitate [who-dd]
60. Hy-b0987
61. Tox21_113637
62. Tox21_301926
63. Bdbm50451094
64. S2532
65. Akos015895292
66. Zinc100004322
67. Zinc100047718
68. Ccg-207947
69. Cs-4478
70. Ascorbyl Palmitate [ep Monograph]
71. Ncgc00255543-01
72. As-12990
73. A0540
74. Sw220267-1
75. D02412
76. D88276
77. A807294
78. Q424521
79. 6-o-palmitoyl-l-ascorbic Acid, Bioxtra, >=99.0% (rt)
80. Ascorbic Acid 6-palmitate, Meets Usp Testing Specifications
81. 6-o-palmitoyl-l-ascorbic Acid, Analytical Reference Material
82. 6-o-palmitoyl-l-ascorbic Acid, Vetec(tm) Reagent Grade, 95%
83. Ascorbyl Palmitate, European Pharmacopoeia (ep) Reference Standard
84. Ascorbyl Palmitate, United States Pharmacopeia (usp) Reference Standard
85. [(2s)-2-[(2r)-4,5-dihydroxy-3-oxo-2-furyl]-2-hydroxy-ethyl] Hexadecanoate
86. 3,4-dihydroxy-5beta-[(s)-2-(hexadecanoyloxy)-1-hydroxyethyl]furan-2(5h)-one
87. Ascorbyl Palmitate, Pharmaceutical Secondary Standard; Certified Reference Material
1. Ascorbyl Palmitic Acid
2. Ondascora
3. Quicifal
4. Cetyl Ascorbate
5. L-ascorbyl Palmitate
| Molecular Weight | 414.5 g/mol |
|---|---|
| Molecular Formula | C22H38O7 |
| XLogP3 | 6.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 18 |
| Exact Mass | 414.26175355 g/mol |
| Monoisotopic Mass | 414.26175355 g/mol |
| Topological Polar Surface Area | 113 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 515 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antimutagenic Agents; Antioxidants
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Ascorbyl palmitate has a vitamin C activity approximately equal to that of L-ascorbic acid. ... Vitamin C is an essential cofactor for prolyl and lysyl hydroxylases, the enzymes involved in the intracellular biosynthesis of collagen.
Cosmetic Ingredient Review; Final Report of the Cosmetic Ingredient Review Expert Panel; Final Report on the Safety Assessment of Ascorbyl Palmitate, Ascorbyl Dipalmitate, Ascorbyl Stearate, Erythorbic Acid, and Sodium Erythorbate; Scientific Regulatory Reference CD-ROM (2006). Cosmetic, Toiletry, and Fragrance Association, Washington D.C.
/Experimental Therapy/ QR-333, a topical compound that contains quercetin, a flavonoid with aldose reductase inhibitor effects, ascorbyl palmitate, and vitamin D(3), was formulated to decrease the oxidative stress that contributes to peripheral diabetic neuropathy and thus alleviate its symptoms. ... This randomized, placebo-controlled, double-blind trial included 34 men and women (21-71 years of age) with Type 1 or 2 diabetes and diabetic neuropathy who applied QR-333 or placebo (2:1 ratio), three times daily for 4 weeks, to each foot where symptoms were experienced. ... QR-333 reduced the severity of numbness, jolting pain, and irritation from baseline values. Improvements were also seen in overall and specific quality-of-life measures. QR-333 was well tolerated. Eleven patients in the QR-333 group reported 23 adverse events (all mild or moderate); 4 in the placebo group reported 5 events (all moderate). One patient who applied QR-333 noted a pricking sensation twice, the only adverse event considered possibly related to study treatment...
PMID:16112498 Valensi P et al; Diabetes Complications 19 (5): 247-53 (2005)
The presence of ascorbyl palmitate in oral supplements contributes to the ascorbic acid content of the supplement and probably helps protect fat-soluble antioxidants in the supplement.
Linus Pauling Institute at Oregon State University; Micronutrient Information Center; The Bioavailability of Different Forms of Vitamin C (Ascorbic Acid). Available from, as of August 8, 2010: https://lpi.oregonstate.edu/infocenter/vitamins/vitaminC/vitCform.html
Antimutagenic Agents
Agents that reduce the frequency or rate of spontaneous or induced mutations independently of the mechanism involved. (See all compounds classified as Antimutagenic Agents.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
When incorporated into the cell membranes of human red blood cells, ascorbyl palmitate has been found to protect them from oxidative damage and to protect alpha-tocopherol (a fat-soluble antioxidant) from oxidation by free radicals. However, the protective effects of ascorbyl palmitate on cell membranes have only been demonstrated in the test tube. Taking ascorbyl palmitate orally probably doesn't result in any significant incorporation into cell membranes because most of it appears to be hydrolyzed (broken apart into palmitate and ascorbic acid) in the human digestive tract before it is absorbed. The ascorbic acid released by the hydrolysis of ascorbyl palmitate appears to be as bioavailable as ascorbic acid alone.
Linus Pauling Institute at Oregon State University; Micronutrient Information Center; The Bioavailability of Different Forms of Vitamin C (Ascorbic Acid). Available from, as of August 8, 2010: https://lpi.oregonstate.edu/infocenter/vitamins/vitaminC/vitCform.html
When applied topically to guinea pigs, ascorbyl palmitate penetrated the skin barrier so that ascorbic acid content in the skin, liver, and blood increased eight-, seven-, and four-fold, respectively, when compared to control animals that did not receive ascorbyl palmitate.
Cosmetic Ingredient Review; Final Report of the Cosmetic Ingredient Review Expert Panel; Final Report on the Safety Assessment of Ascorbyl Palmitate, Ascorbyl Dipalmitate, Ascorbyl Stearate, Erythorbic Acid, and Sodium Erythorbate; Scientific Regulatory Reference CD-ROM (2006). Cosmetic, Toiletry, and Fragrance Association, Washington D.C.
(14)C-Ascorbyl palmitate was applied to the skin of scorbutic (affected by scurvy) guinea pigs. Following the topical application, ascorbic acid concentrations in the skin, liver, kidneys, and blood were four to eight times greater than in the control.
Cosmetic Ingredient Review; Final Report of the Cosmetic Ingredient Review Expert Panel; Final Report on the Safety Assessment of Ascorbyl Palmitate, Ascorbyl Dipalmitate, Ascorbyl Stearate, Erythorbic Acid, and Sodium Erythorbate; Scientific Regulatory Reference CD-ROM (2006). Cosmetic, Toiletry, and Fragrance Association, Washington D.C.
Ascorbyl palmitate dissolved in a sodium taurocholate solution was hydrolyzed by homogenates of the liver, pancreas, and intestines of guinea pigs. Approximately 80% of ascorbyl palmitate was hydrolyzed to free ascorbic acid by homogenates of the small intestine and pancreas. ... Ascorbyl palmitate (the equivalent of 20 mg of ascorbic acid) was orally administered to guinea pigs, and the amount of free ascorbic acid excreted in the urine was measured. Greater amounts of acid were excreted at 0-24 hours than at 24-48 hours. A similar trend was found in these organs of free ascorbic acid content when L-ascorbic acid was administered instead, but a reverse tendency was observed with ascorbyl palmitate.
Cosmetic Ingredient Review; Final Report of the Cosmetic Ingredient Review Expert Panel; Final Report on the Safety Assessment of Ascorbyl Palmitate, Ascorbyl Dipalmitate, Ascorbyl Stearate, Erythorbic Acid, and Sodium Erythorbate; Scientific Regulatory Reference CD-ROM (2006). Cosmetic, Toiletry, and Fragrance Association, Washington D.C.
Vitamin C (ascorbic acid) is a non-enzymatic antioxidant important in protecting the lung against oxidative damage and is decreased in lung lining fluid of horses with airway inflammation. To examine possible therapeutic regimens in a species with ascorbate-synthesising capacity, ... Te effects of oral supplementation of two forms of ascorbic acid, (each equivalent to 20 mg ascorbic acid per kg body weight) on the pulmonary and systemic antioxidant status of six healthy ponies in a 3 x 3 Latin square design. Two weeks supplementation with ascorbyl palmitate significantly increased mean plasma ascorbic acid concentrations compared to control (29 +/-- 5 and 18 +/- 7 umol/L, respectively; p < 0.05). Calcium ascorbyl-2-monophosphate, a more stable form of ascorbic acid, also increased mean plasma ascorbic acid concentrations, but not significantly (23 +/- 1 umol/L; p = 0.07). The concentration of ascorbic acid in bronchoalveolar lavage fluid increased in five out of six ponies following supplementation with either ascorbyl palmitate or calcium ascorbyl-2-monophosphate compared with control (30 +/- 10, 25 +/- 4 and 18 +/- 8 umol/L, respectively; p < 0.01). Neither supplement altered the concentration of glutathione, uric acid or alpha-tocopherol in plasma or bronchoalveolar lavage fluid. In conclusion, the concentration of lung lining fluid ascorbic acid is increased following ascorbic acid supplementation (20 mg/kg body weight) in an ascorbate-synthesising species.
PMID:12747741 Deaton CM et al; Free Radic Res 37 (4): 461-7 (2003)
It has been known that solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) have occlusive effects, but ascorbyl palmitate (AP) incorporation moisturized skin significantly better than placebo in short-term (p < 0.001) and long-term trials (p < 0.01) for both SLN and NLC. In the second part of the study, SLN and NLC were found to sustain the penetration of AP through excised human skin about 1/2 and 2/3 times compared to NE (p < 0.001 and p < 0.01), respectively...
PMID:16259122 Uner M et al; Pharmazie 60 (10): 751-5 (2005)
6-O-Palmitoyl-L-ascorbic acid dissolved in a sodium taurocholate solution was hydrolyzed by homogenates of the pancreas, liver, and intestines of guinea pigs.
INAGAKI C ET AL; VITAMINS 37(2) 147 (1968)
... Whether L-ascorbic acid 6-palmitate (AAP), an amphipathic derivative of AA, has chemopreventive effects /was examined/ using a gap-junctional intercellular communication (GJIC) model. AAP and ascorbic acid (AA) exhibited dose-dependent free radical-scavenging activities and inhibited hydrogen peroxide (H(2)O(2))-induced intracellular reactive oxygen species (ROS) production in normal rat liver epithelial cells. Unexpectedly, however, AAP did not protect against the inhibition of GJIC induced by H(2)O(2); instead, it inhibited GJIC synergistically with H(2)O(2). AAP inhibited GJIC in a dose-dependent and reversible manner. This inhibitory effect was not due to the conjugated lipid structure of AAP, as treatment with palmitic acid alone failed to inhibit GJIC under the same conditions. The inhibition of GJIC by AAP was restored in the presence of mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) kinase (MEK) inhibitor U0126, but not in the presence of other signal inhibitors and antioxidant (PKC inhibitors, EGFR inhibitor, NADPH oxidase inhibitor, catalase, vitamin E, or AA), indicating the critical involvement of MEK signaling in the GJIC inhibitory activity of AAP. Phosphorylation of ERK and connexin 43 (Cx43) was observed following AAP treatment, and this was reversed by U0126. These results suggest that the AAP-induced inhibition of GJIC is mediated by the phosphorylation of Cx43 via activation of the MEK-ERK pathway.
PMID:19026667 Lee KM et al; Mutat Res 660 (1-2): 51-6 (2009)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
20
PharmaCompass offers a list of Ascorbyl Palmitate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ascorbyl Palmitate manufacturer or Ascorbyl Palmitate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ascorbyl Palmitate manufacturer or Ascorbyl Palmitate supplier.
PharmaCompass also assists you with knowing the Ascorbyl Palmitate API Price utilized in the formulation of products. Ascorbyl Palmitate API Price is not always fixed or binding as the Ascorbyl Palmitate Price is obtained through a variety of data sources. The Ascorbyl Palmitate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ascorbyl Palmitate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ascorbyl Palmitate, including repackagers and relabelers. The FDA regulates Ascorbyl Palmitate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ascorbyl Palmitate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Ascorbyl Palmitate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Ascorbyl Palmitate supplier is an individual or a company that provides Ascorbyl Palmitate active pharmaceutical ingredient (API) or Ascorbyl Palmitate finished formulations upon request. The Ascorbyl Palmitate suppliers may include Ascorbyl Palmitate API manufacturers, exporters, distributors and traders.
click here to find a list of Ascorbyl Palmitate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Ascorbyl Palmitate CEP of the European Pharmacopoeia monograph is often referred to as a Ascorbyl Palmitate Certificate of Suitability (COS). The purpose of a Ascorbyl Palmitate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Ascorbyl Palmitate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Ascorbyl Palmitate to their clients by showing that a Ascorbyl Palmitate CEP has been issued for it. The manufacturer submits a Ascorbyl Palmitate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Ascorbyl Palmitate CEP holder for the record. Additionally, the data presented in the Ascorbyl Palmitate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Ascorbyl Palmitate DMF.
A Ascorbyl Palmitate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Ascorbyl Palmitate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Ascorbyl Palmitate suppliers with CEP (COS) on PharmaCompass.
A Ascorbyl Palmitate written confirmation (Ascorbyl Palmitate WC) is an official document issued by a regulatory agency to a Ascorbyl Palmitate manufacturer, verifying that the manufacturing facility of a Ascorbyl Palmitate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Ascorbyl Palmitate APIs or Ascorbyl Palmitate finished pharmaceutical products to another nation, regulatory agencies frequently require a Ascorbyl Palmitate WC (written confirmation) as part of the regulatory process.
click here to find a list of Ascorbyl Palmitate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Ascorbyl Palmitate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Ascorbyl Palmitate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Ascorbyl Palmitate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Ascorbyl Palmitate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Ascorbyl Palmitate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Ascorbyl Palmitate suppliers with NDC on PharmaCompass.
Ascorbyl Palmitate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ascorbyl Palmitate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ascorbyl Palmitate GMP manufacturer or Ascorbyl Palmitate GMP API supplier for your needs.
A Ascorbyl Palmitate CoA (Certificate of Analysis) is a formal document that attests to Ascorbyl Palmitate's compliance with Ascorbyl Palmitate specifications and serves as a tool for batch-level quality control.
Ascorbyl Palmitate CoA mostly includes findings from lab analyses of a specific batch. For each Ascorbyl Palmitate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ascorbyl Palmitate may be tested according to a variety of international standards, such as European Pharmacopoeia (Ascorbyl Palmitate EP), Ascorbyl Palmitate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ascorbyl Palmitate USP).
