
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Weekly News Recap #Phispers

1. (11c)abt-627
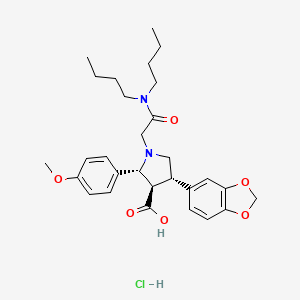
2. 2-(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-(((dibutylamino)carbonyl)methyl)pyrrolidine-3-carboxylic Acid
3. A 127722
4. A 127722.5
5. A 147627
6. A-127722
7. A-127722.5
8. A-147627
9. A127722
10. A127722.5
11. A147627
12. Abt 627
13. Abt-627
14. Abt627
15. Atrasentan
16. Xinlay
1. 195733-43-8
2. Atrasentan Hcl
3. Atrasentan (hydrochloride)
4. Atrasentan Hydrochloride [usan]
5. Abbot-147627
6. E4g31x93za
7. 3-pyrrolidinecarboxylicacid,4-(1,3-benzodioxol-5-yl)-1-[2-(dibutylamino)-2-oxoethyl]-2-(4-methoxyphenyl)-,hydrochloride (1:1), (2r,3r,4s)-
8. 195733-43-8 (hcl)
9. A-147627.1
10. Atrasentan Hydrochloride (usan)
11. Abbott 147627
12. Unii-e4g31x93za
13. (2r,3r,4s)-1-((dibutylcarbamoyl)methyl)-2-(p-methoxyphenyl)-4-(3,4-(methylenedioxy)phenyl)-3-pyrrolidinecarboxylic Acid, Monohydrochloride
14. (2r,3r,4s)-4-(benzo[d][1,3]dioxol-5-yl)-1-(2-(dibutylamino)-2-oxoethyl)-2-(4-methoxyphenyl)pyrrolidine-3-carboxylic Acid Hydrochloride
15. (2r,3r,4s)-4-(benzo[d][1,3]dioxol-5-yl)-1-(2-(dibutylamino)-2-oxoethyl)-2-(4-methoxyphenyl)pyrrolidine-3-carboxylic Acid Hydrochloride.
16. A 147627.1
17. D03009
18. (2r,3r,4s)-4-(1,3-benzodioxol-5-yl)-1-[2-(dibutylamino)-2-oxoethyl]-2-(4-methoxyphenyl)pyrrolidine-3-carboxylic Acid Hydrochloride
19. Chembl2106068
20. Dtxsid20173240
21. Ex-a4175
22. Atrasentan Hydrochloride [mi]
23. Hy-15403a
24. Mfcd13194884
25. Cs-1373
26. Atrasentan Hydrochloride [mart.]
27. Atrasentan Hydrochloride [who-dd]
28. Bs-16407
29. Atrasentan Hydrochloride, >=98% (hplc)
30. D82853
31. 733a438
32. A930316
33. Abt-627;abt627;abt 627;abbott 147627
34. Q27276859
35. (2r,3r,4s)-(+)-2-(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-(n,n-di(n-butyl)aminocarbonylmethyl)-pyrrolidine-3-carboxylic Acid Hydrochloride Salt
36. (2r,3r,4s)-4-(1,3-benzodioxol-5-yl)-1-[2-(dibutylamino)-2-oxoethyl]-2-(4-methoxyphenyl)pyrrolidine-3-carboxylic Acid;hydrochloride
37. (2r,3r,4s)-4-(benzo[d][1,3]dioxol-5-yl)-1-(2-(dibutylamino)-2-oxoethyl)-2-(4-methoxyphenyl)pyrrolidine-3-carboxylicacidhydrochloride
38. 3-pyrrolidinecarboxylic Acid, 4-(1,3-benzodioxol-5-yl)-1-(2-(dibutylamino)-2-oxoethyl)-2-(4-methoxyphenyl)-, Monohydrochloride, (2r,3r,4s)-
39. 3-pyrrolidinecarboxylic Acid, 4-(1,3-benzodioxol-5-yl)-1-(2-(dibutylamino)-2-oxoethyl)-2-(4-methoxyphenyl)-, Monohydrochloride, (2r-(2.alpha.,3.beta.,4.alpha.))
40. 3-pyrrolidinecarboxylic Acid, 4-(1,3-benzodioxol-5-yl)-1-(2-(dibutylamino)-2-oxoethyl)-2-(4-methoxyphenyl)-, Monohydrochloride, (2r-(2alpha,3beta,4alpha))
| Molecular Weight | 547.1 g/mol |
|---|---|
| Molecular Formula | C29H39ClN2O6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 12 |
| Exact Mass | 546.2496647 g/mol |
| Monoisotopic Mass | 546.2496647 g/mol |
| Topological Polar Surface Area | 88.5 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 734 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Treatment of nephropathy
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Endothelin A Receptor Antagonists
Compounds and drugs that bind to and inhibit or block the activation of ENDOTHELIN A RECECPTORS. (See all compounds classified as Endothelin A Receptor Antagonists.)
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17686
Submission : 2004-09-05
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Chinook Therapeutics in-license of atrasentan provides a unique opportunity for Chinook to add a potential new drug to its precision medicine portfolio for the treatment of rare CKD conditions.
Lead Product(s): Atrasentan
Therapeutic Area: Nephrology Brand Name: CHK-01
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Chinook Therapeutics
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Licensing Agreement October 01, 2020
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Atrasentan
Therapeutic Area : Nephrology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Chinook Therapeutics
Deal Size : Undisclosed
Deal Type : Licensing Agreement
Details : Chinook Therapeutics in-license of atrasentan provides a unique opportunity for Chinook to add a potential new drug to its precision medicine portfolio for the treatment of rare CKD conditions.
Product Name : CHK-01
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
October 01, 2020
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The partnership aims to distribute Novartis' Vanrafia (atrasentan HCl) through CareMed. It is indicated for the treatment of proteinuria in adults with primary immunoglobulin A nephropathy.
Lead Product(s): Atrasentan
Therapeutic Area: Nephrology Brand Name: Vanrafia
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: CareMed
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership April 07, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Atrasentan
Therapeutic Area : Nephrology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : CareMed
Deal Size : Undisclosed
Deal Type : Partnership
CareMed Chosen As Specialty Pharmacy Partner For VANRAFIA
Details : The partnership aims to distribute Novartis' Vanrafia (atrasentan HCl) through CareMed. It is indicated for the treatment of proteinuria in adults with primary immunoglobulin A nephropathy.
Product Name : Vanrafia
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
April 07, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vanrafia (atrasentan HCl) is an investigational oral endothelin A receptor antagonist (ERA), which is approved for IgAN and early-stage development for other rare kidney diseases.
Lead Product(s): Atrasentan
Therapeutic Area: Nephrology Brand Name: Vanrafia
Study Phase: Approved FDFProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 02, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Atrasentan
Therapeutic Area : Nephrology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Novartis Gets FDA Approval for Vanrafia® to Reduce Proteinuria in IgAN
Details : Vanrafia (atrasentan HCl) is an investigational oral endothelin A receptor antagonist (ERA), which is approved for IgAN and early-stage development for other rare kidney diseases.
Product Name : Vanrafia
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
April 02, 2025

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
CHK-01 (atrasentan HCl) is an investigational oral endothelin A receptor antagonist (ERA), currently in Phase III development for IgAN and early-stage development for other rare kidney diseases
Lead Product(s): Atrasentan
Therapeutic Area: Nephrology Brand Name: CHK-01
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable May 25, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Atrasentan
Therapeutic Area : Nephrology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Novartis Shows Proteinuria Reduction Data from Atrasentan Phase III Trial in IgAN
Details : CHK-01 (atrasentan HCl) is an investigational oral endothelin A receptor antagonist (ERA), currently in Phase III development for IgAN and early-stage development for other rare kidney diseases
Product Name : CHK-01
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
May 25, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Novartis will acquire SanReno's pipeline, gaining exclusive rights for Greater China and Singapore for late-stage IgAN assets, ABT-627 (oral ERA antagonist) and zigakibart (monoclonal antibody).
Lead Product(s): Atrasentan
Therapeutic Area: Nephrology Brand Name: ABT-627
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Novartis Pharmaceuticals Corporation
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Acquisition May 01, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Atrasentan
Therapeutic Area : Nephrology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Novartis Pharmaceuticals Corporation
Deal Size : Undisclosed
Deal Type : Acquisition
SanReno Acquired By Novartis for Kidney Disease Therapeutics
Details : Novartis will acquire SanReno's pipeline, gaining exclusive rights for Greater China and Singapore for late-stage IgAN assets, ABT-627 (oral ERA antagonist) and zigakibart (monoclonal antibody).
Product Name : ABT-627
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
May 01, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Through the acquisition, Novartis expands its portfolio with chinook's renal pipeline having 2 assets, CHK-01 (atrasentan hydrochloride), an oral endothelin A receptor antagonist and BION-1301 (zigakibart), an anti-APRIL monoclonal antibody, for Immunoglobulin A Nephropathy.
Lead Product(s): Atrasentan
Therapeutic Area: Nephrology Brand Name: CHK-01
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Novartis Pharmaceuticals Corporation
Deal Size: $3,500.0 million Upfront Cash: $3,500.0 million
Deal Type: Acquisition December 06, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Atrasentan
Therapeutic Area : Nephrology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Novartis Pharmaceuticals Corporation
Deal Size : $3,500.0 million
Deal Type : Acquisition
Details : Through the acquisition, Novartis expands its portfolio with chinook's renal pipeline having 2 assets, CHK-01 (atrasentan hydrochloride), an oral endothelin A receptor antagonist and BION-1301 (zigakibart), an anti-APRIL monoclonal antibody, for Immunogl...
Product Name : CHK-01
Product Type : Other Small Molecule
Upfront Cash : $3,500.0 million
December 06, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Through the acquisition, Novartis expands its portfolio with chinook's renal pipeline having 2 assets, CHK-01 (atrasentan hydrochloride), an oral endothelin A receptor antagonist and BION-1301 (zigakibart), an anti-APRIL monoclonal antibody, for Immunoglobulin A Nephropathy.
Lead Product(s): Atrasentan
Therapeutic Area: Nephrology Brand Name: CHK-01
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Novartis Pharmaceuticals Corporation
Deal Size: $3,500.0 million Upfront Cash: $3,500.0 million
Deal Type: Acquisition November 08, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Atrasentan
Therapeutic Area : Nephrology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Novartis Pharmaceuticals Corporation
Deal Size : $3,500.0 million
Deal Type : Acquisition
Novartis Completes Acquisition of Chinook Therapeutics
Details : Through the acquisition, Novartis expands its portfolio with chinook's renal pipeline having 2 assets, CHK-01 (atrasentan hydrochloride), an oral endothelin A receptor antagonist and BION-1301 (zigakibart), an anti-APRIL monoclonal antibody, for Immunogl...
Product Name : CHK-01
Product Type : Other Small Molecule
Upfront Cash : $3,500.0 million
November 08, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
CHK-01 (atrasentan hydrochloride) is an investigational oral endothelin A receptor antagonist (ERA), currently in Phase III development for IgAN and early-stage development for other rare kidney diseases
Lead Product(s): Atrasentan
Therapeutic Area: Nephrology Brand Name: CHK-01
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Atrasentan
Therapeutic Area : Nephrology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : CHK-01 (atrasentan hydrochloride) is an investigational oral endothelin A receptor antagonist (ERA), currently in Phase III development for IgAN and early-stage development for other rare kidney diseases
Product Name : CHK-01
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
October 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
CHK-01 (atrasentan hydrochloride), an oral endothelin A receptor antagonist is being evaluated phase 3 clinical trials for proteinuria reduction in patients with IgA nephropathy.
Lead Product(s): Atrasentan
Therapeutic Area: Nephrology Brand Name: CHK-01
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable October 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Atrasentan
Therapeutic Area : Nephrology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : CHK-01 (atrasentan hydrochloride), an oral endothelin A receptor antagonist is being evaluated phase 3 clinical trials for proteinuria reduction in patients with IgA nephropathy.
Product Name : CHK-01
Product Type : Other Small Molecule
Upfront Cash : Inapplicable
October 30, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Chinook intends to use the net proceeds from this offering to continue its phase 3 ALIGN and phase 2 AFFINITY trials of atrasentan, fund a phase 3 clinical trial of BION-1301, continue development of CHK-336 and prepare for the potential commercial launch of atrasentan.
Lead Product(s): Atrasentan
Therapeutic Area: Nephrology Brand Name: CHK-01
Study Phase: Phase IIIProduct Type: Other Small Molecule
Sponsor: SVB Securities
Deal Size: $105.0 million Upfront Cash: Undisclosed
Deal Type: Public Offering May 25, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Atrasentan
Therapeutic Area : Nephrology
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : SVB Securities
Deal Size : $105.0 million
Deal Type : Public Offering
Chinook Therapeutics Announces Pricing of a $105 Million Public Offering
Details : Chinook intends to use the net proceeds from this offering to continue its phase 3 ALIGN and phase 2 AFFINITY trials of atrasentan, fund a phase 3 clinical trial of BION-1301, continue development of CHK-336 and prepare for the potential commercial launc...
Product Name : CHK-01
Product Type : Other Small Molecule
Upfront Cash : Undisclosed
May 25, 2022

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

ABOUT THIS PAGE
25
PharmaCompass offers a list of Atrasentan API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Atrasentan manufacturer or Atrasentan supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Atrasentan manufacturer or Atrasentan supplier.
PharmaCompass also assists you with knowing the Atrasentan API Price utilized in the formulation of products. Atrasentan API Price is not always fixed or binding as the Atrasentan Price is obtained through a variety of data sources. The Atrasentan Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Atrasentan manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Atrasentan, including repackagers and relabelers. The FDA regulates Atrasentan manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Atrasentan API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Atrasentan manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Atrasentan supplier is an individual or a company that provides Atrasentan active pharmaceutical ingredient (API) or Atrasentan finished formulations upon request. The Atrasentan suppliers may include Atrasentan API manufacturers, exporters, distributors and traders.
click here to find a list of Atrasentan suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Atrasentan DMF (Drug Master File) is a document detailing the whole manufacturing process of Atrasentan active pharmaceutical ingredient (API) in detail. Different forms of Atrasentan DMFs exist exist since differing nations have different regulations, such as Atrasentan USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Atrasentan DMF submitted to regulatory agencies in the US is known as a USDMF. Atrasentan USDMF includes data on Atrasentan's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Atrasentan USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Atrasentan suppliers with USDMF on PharmaCompass.
Atrasentan Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Atrasentan GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Atrasentan GMP manufacturer or Atrasentan GMP API supplier for your needs.
A Atrasentan CoA (Certificate of Analysis) is a formal document that attests to Atrasentan's compliance with Atrasentan specifications and serves as a tool for batch-level quality control.
Atrasentan CoA mostly includes findings from lab analyses of a specific batch. For each Atrasentan CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Atrasentan may be tested according to a variety of international standards, such as European Pharmacopoeia (Atrasentan EP), Atrasentan JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Atrasentan USP).
