
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
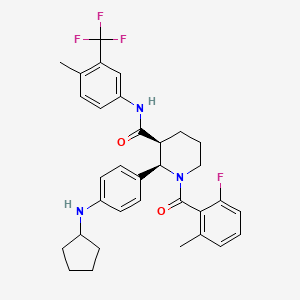

1. Ccx168
1. 1346623-17-3
2. Ccx168
3. Avacopan [inn]
4. Avacopan [usan]
5. Ccx-168
6. O880nm097t
7. (2r,3s)-2-(4-(cyclopentylamino)phenyl)-1-(2-fluoro-6-methylbenzoyl)-n-(4-methyl-3-(trifluoromethyl)phenyl)piperidine-3-carboxamide
8. (2r,3s)-2-[4-(cyclopentylamino)phenyl]-1-(2-fluoro-6-methylbenzoyl)-n-[4-methyl-3-(trifluoromethyl)phenyl]piperidine-3-carboxamide
9. Tavneos
10. 3-piperidinecarboxamide, 2-(4-(cyclopentylamino)phenyl)-1-(2-fluoro-6-methylbenzoyl)-n-(4-methyl-3-(trifluoromethyl)phenyl)-, (2r,3s)-
11. Ccx168; Avacopan
12. Avacopan [jan]
13. Avacopan [usan:inn]
14. Avacopan [who-dd]
15. Avacopan (jan/usan/inn)
16. Unii-o880nm097t
17. Avacopan [orange Book]
18. Gtpl9450
19. Schembl2567144
20. Chembl3989871
21. C33h35f4n3o2
22. Dtxsid701102660
23. Ex-a2605
24. Mfcd28502293
25. At30195
26. Cs-6888
27. Db15011
28. Ac-35654
29. Hy-17627
30. J3.663.585a
31. D11093
32. Q27285470
33. (2r,3s)-2-[4-(cyclopentylamino)phenyl]-1-(2-fluoro-6-methyl-benzoyl)-n-[4-methyl-3-(trifluoromethyl)phenyl]piperidine-3-carboxamide
34. (2r,3s)-2-[4-(cyclopentylamino)phenyl]-1-(2-fluoro-6-methylbenzene-1-carbonyl)-n-[4-methyl-3-(trifluoromethyl)phenyl]piperidine-3-carboxamide
35. (2r,3s)-2-[4-(cyclopentylamino)phenyl]-1-(2-fluoro-6-methylbenzoyl)-n-[4-methyl-3-(trifluoromethyl)phenyl]-3-piperidinecarboxamide
36. Efd
| Molecular Weight | 581.6 g/mol |
|---|---|
| Molecular Formula | C33H35F4N3O2 |
| XLogP3 | 7.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 6 |
| Exact Mass | 581.26654002 g/mol |
| Monoisotopic Mass | 581.26654002 g/mol |
| Topological Polar Surface Area | 61.4 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 918 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Avacopan is indicated for the adjunctive treatment of adult patients with severe active anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis (granulomatosis with polyangiitis and microscopic polyangiitis; GPA/MPA) in combination with standard therapy including glucocorticoids. Avacopan does not eliminate the need for glucocorticoids.
Tavneos, in combination with a rituximab or cyclophosphamide regimen, is indicated for the treatment of adult patients with severe, active granulomatosis with polyangiitis (GPA) or microscopic polyangiitis (MPA).
Avacopan is a complement 5a receptor (C5aR) antagonist that blocks C5a-induced upregulation of C11b (integrin alpha M) on neutrophils and inhibits C5a-mediated neutrophil activation and migration. Avacopan has been associated with hypersensitivity reactions, including angioedema, and hepatotoxicity, as evidenced by elevated liver transaminases. Likely due to its effect on the complement pathway, avacopan has also been associated with hepatitis B virus reactivation and serious infections, which should be monitored for as appropriate.
L04
Absorption
In AAV patients receiving 30 mg avacopan twice daily, avacopan had a Cmax of 349 169 ng/mL and an AUC0-12hr of 3466 1921 ng\*h/mL. On this dosing scheme, steady-state plasma concentrations are reached by 13 weeks with a roughly 4-fold accumulation. Co-administration of 30 mg with a high-fat meal increased the Cmax by ~8%, the AUC by ~72%, and delayed the Tmax by four hours (from two hours).
Route of Elimination
Avacopan is mainly eliminated in feces, with smaller amounts present in the urine. Following oral administration of the radiolabeled drug, roughly 77% (7% as unchanged avacopan) was recovered in feces while 10% (<0.1% unchanged) was recovered in urine.
Volume of Distribution
Avacopan has an apparent volume of distribution of 345 L.
Clearance
Avacopan has an estimated total apparent body clearance (CL/F) of 16.3 L/h.
Avacopan is metabolized primarily by CYP3A4. The major circulating M1 metabolite, a mono-hydroxylated form of avacopan, represents ~12% of drug plasma levels and acts as a C5aR antagonist with similar efficacy to avacopan itself.
A single 30 mg dose of avacopan given with food to healthy subjects resulted in mean elimination half-lives of 97.6 and 55.6 hours for avacopan and its M1 metabolite, respectively.
Anti-neutrophil cytoplasmic (auto)antibody (ANCA)-associated vasculitis (AAV) is considered a "pauci-immune" form of systemic small-vessel vasculitis accompanied by the presence of ANCAs in the serum. The full spectrum of AAV includes granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), eosinophilic granulomatosis with polyangiitis (EGPA), and drug-induced AAV. AAV may be associated with necrotizing and crescentic glomerulonephritis (NCGN). Of the various known ANCAs, the major antigens are myeloperoxidase (MPO) and proteinase 3 (PR3/myeloblastin). The pathophysiology giving rise to AAV is complex, though a working model has been proposed. An initial trigger, such as infection, causes differentiation of naive T cells into TH17 helper T cells that induce the release from macrophages of pro-inflammatory cytokines (e.g., TNF- and IL-1), which prime neutrophils. Concurrently, the anaphylatoxin C5a is produced through activation of the alternative complement pathway, which also primes neutrophils through binding to the C5a receptor (C5aR; CD88). Primed neutrophils undergo physiological changes, including upregulating the display of ANCA antigens on their surface. Circulating ANCAs bind to displayed ANCA antigens on the surface of neutrophils; simultaneously, the Fc region of these ANCAs is recognized by Fc receptors on other neutrophils, resulting in excessive neutrophil activation. Activated neutrophils form NETs (neutrophil extracellular traps), which induce tissue damage and vasculitis. MPO/PR3 in NETs induces further ANCA production through dendritic cell- and CD4+ T cell-mediated activation of B cells, further exacerbating the condition. A role for complement was not initially considered in AAV due to a lack of excessive complement or immunoglobulin deposition in AAV lesions. However, extensive molecular studies confirmed a significant role for the alternative complement pathway, acting through C3 and C5, in the pathogenesis of AAV. The C5a fragment, generated by C5 cleavage, can bind to both the C5aR and C5a-like receptor (C5L2) on the surface of neutrophils; C5aR binding is associated with AAV while C5L2 binding has a protective effect. As the alternative complement pathway is self-sustaining in the absence of down-regulation by specific proteins, it is likely a significant driver of AAV. Furthermore, neutrophils activated by C5a release reactive oxygen species, properdin, and other molecules that stimulate the complement pathway leading to the production of more C5a in a vicious cycle. Avacopan (CCX168) is a specific C5aR receptor allosteric antagonist that inhibits C5a-mediated neutrophil activation both _in vitro_ and _in vivo_. By inhibiting the C5a/C5aR axis, avacopan should have minimal effects on the formation of the membrane attack complex (which includes C5b) and therefore little effect on the innate immune response in treated patients.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 40159
Submission : 2024-06-29
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
74
PharmaCompass offers a list of Avacopan API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Avacopan manufacturer or Avacopan supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Avacopan manufacturer or Avacopan supplier.
PharmaCompass also assists you with knowing the Avacopan API Price utilized in the formulation of products. Avacopan API Price is not always fixed or binding as the Avacopan Price is obtained through a variety of data sources. The Avacopan Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Avacopan manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Avacopan, including repackagers and relabelers. The FDA regulates Avacopan manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Avacopan API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Avacopan manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Avacopan supplier is an individual or a company that provides Avacopan active pharmaceutical ingredient (API) or Avacopan finished formulations upon request. The Avacopan suppliers may include Avacopan API manufacturers, exporters, distributors and traders.
click here to find a list of Avacopan suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Avacopan DMF (Drug Master File) is a document detailing the whole manufacturing process of Avacopan active pharmaceutical ingredient (API) in detail. Different forms of Avacopan DMFs exist exist since differing nations have different regulations, such as Avacopan USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Avacopan DMF submitted to regulatory agencies in the US is known as a USDMF. Avacopan USDMF includes data on Avacopan's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Avacopan USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Avacopan suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Avacopan Drug Master File in Japan (Avacopan JDMF) empowers Avacopan API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Avacopan JDMF during the approval evaluation for pharmaceutical products. At the time of Avacopan JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Avacopan suppliers with JDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Avacopan as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Avacopan API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Avacopan as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Avacopan and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Avacopan NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Avacopan suppliers with NDC on PharmaCompass.
Avacopan Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Avacopan GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Avacopan GMP manufacturer or Avacopan GMP API supplier for your needs.
A Avacopan CoA (Certificate of Analysis) is a formal document that attests to Avacopan's compliance with Avacopan specifications and serves as a tool for batch-level quality control.
Avacopan CoA mostly includes findings from lab analyses of a specific batch. For each Avacopan CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Avacopan may be tested according to a variety of international standards, such as European Pharmacopoeia (Avacopan EP), Avacopan JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Avacopan USP).
