
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Europe

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
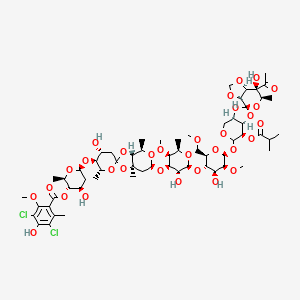

1. 11051-71-1
2. Surmax
3. Avilamycine [inn-french]
4. Avilamycinum [inn-latin]
5. Avilamycina [inn-spanish]
6. Avilamycina
7. Avilamycine
8. Avilamycinum
9. Ly 048740
10. Hsdb 7029
11. Ly 048 740
12. Avilamycin [usan:inn:ban]
13. Unii-720wdx56d3
14. Dtxsid40891398
15. 720wdx56d3
16. Db11375
| Molecular Weight | 1404.2 g/mol |
|---|---|
| Molecular Formula | C61H88Cl2O32 |
| XLogP3 | 0.6 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 32 |
| Rotatable Bond Count | 20 |
| Exact Mass | 1402.4635760 g/mol |
| Monoisotopic Mass | 1402.4635760 g/mol |
| Topological Polar Surface Area | 385 Ų |
| Heavy Atom Count | 95 |
| Formal Charge | 0 |
| Complexity | 2660 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 29 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Bacterial Agents
National Library of Medicine's Medical Subject Headings. Avilamycin. Online file (MeSH, 2015). Available from, as of August 19, 2015: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
MEDICATION (VET): Avilamycin is mainly active against Gram-positive bacteria, including Bacillus spp., Clostridium spp., Corynebacterium bovis, Enterococcus spp., Lactobacillus spp., Listeria monocytogenes, Micrococcus luteus, Staphylococcus aureus and Streptococcus spp. Avilamycin is intended for use as a veterinary medicine in chickens, turkeys, pigs and rabbits to control bacterial enteric infections.
WHO/FAO; Seventieth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 61: Avilamycin p.4 (2009). Available from, as of October 16, 2015: https://www.inchem.org/pages/jecfa.html
MEDICATION (VET): For the reduction in incidence and overall severity of diarrhea in the presence of pathogenic Escherichia coli in groups of weaned pigs. /Included in US product label/
NIH; DailyMed. Current Medication Information for Kavault (Avilamycin) Granule (Updated: August 2015). Available from, as of October 29, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=52282356-9ef2-46ea-a002-a0872b33e0c3
Avilamycin has not been demonstrated to be effective in pigs showing clinical signs of diarrhea prior to the start of medication. The safety of avilamycin has not been established in swine intended for breeding purposes.
NIH; DailyMed. Current Medication Information for Kavault (Avilamycin) Granule (Updated: August 2015). Available from, as of October 29, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=52282356-9ef2-46ea-a002-a0872b33e0c3
To assure responsible antimicrobial drug use in pigs, do not administer to pigs 14 weeks of age or older. Do not administer medicated feed containing avilamycin to pigs for more than a lifetime total of 42 days.
NIH; DailyMed. Current Medication Information for Kavault (Avilamycin) Granule (Updated: August 2015). Available from, as of October 29, 2015: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=52282356-9ef2-46ea-a002-a0872b33e0c3
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
(14)C-Avilamycin was fed to growing swine at a level of 60-80 ppm (1.5-2 times the recommended use level), and tissues were assayed for radioactivity (RA). At a practical zero withdrawal swine fed 60 ppm of uniformly labeled (U-14)C-avilamycin for 14 days had RA residues of 0.14, 0.66, 0.34, and 0.55 ppm in muscle, liver, kidney, and fat, respectively. Swine fed 80 ppm of (14)C-avilamycin labeled in the dichloroisoeverninic acid portion had residues 3-5 times lower, indicative that most of the residue was derived from the oligosaccharide portion of avilamycin. The primary metabolite in liver and feces was flambic acid. Most of the RA in fat from swine fed (U-14)C-avilamycin was in the fatty acids. (14)C-Avilamycin was excreted rapidly and nearly quantitatively by swine, with 5% of the dose in the murine and the remainder in feces. The excretion pattern and metabolic profile of (14)C-avilamycin in the rat were similar to swine.
Magnussen JD et al; J Agric Food Chem 39 (2): 306-310 (1991)
Seven female and four to five male starter pigs weighing 7-12 kg were fed standard diets containing 20 mg avilamycin/kg in three different product forms (crystalline, micronized and non-micronized) for 6 days. The feces collected from pigs that were fed crystalline, micronized and non-micronized product had microbiologically active residues that represented 2.0%, 4.5% and 15.0%, respectively, of the residues of avilamycin and its degradation products, as determined by gas chromatographic assays. The faeces contained an average of 0.94, 2.28 and 8.45 ug of microbiologically active residues per gram for pigs fed crystalline, micronized and non-micronized avilamycin, respectively. The gas chromatographic assay, which determined the total residues of avilamycin plus any degradation products that hydrolyse to DIA, indicated that the faeces contained 43.3, 40.1 and 43.4 ug/g for pigs fed the crystalline, micronized and non-micronized product forms, respectively.
WHO/FAO; Seventieth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 61: Avilamycin p.6 (2009). Available from, as of October 16, 2015: https://www.inchem.org/pages/jecfa.html
... Two crossbred female pigs weighing approximately 40 kg each received 0.9 kg of feed containing unlabelled avilamycin at 60 mg activity/kg in the diet twice daily for 7 days. After being fed the unlabelled drug, each pig received a one-time dose of 120 mg of (14)C-avilamycin (9.3 kBq/mg) incorporated into 450 g of diet. After the consumption of the diet containing (14)C-avilamycin, the animals were given an additional 450 g of unmedicated diet. The female pigs were then fed twice daily with 0.9 kg of unmedicated feed for the duration of the experiment. Most of the (14)C residues in both pigs were excreted in the first 4 days, with over 91% eliminated on days 2 and 3. The peak excretion of (14)C residues in urine occurred in the first 24-hr collection period, with 2.75% and 3.30% recovery for the two animals. During the 9-day collection period, the two pigs excreted 96.9% and 99.0%, respectively, of the total dose administered. An average of 93.4% of the excreted dose was found in the feces, and 4.54% was found in the urine.
WHO/FAO; Seventieth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 61: Avilamycin p.5 (2009). Available from, as of October 16, 2015: https://www.inchem.org/pages/jecfa.html
Six male and six female 7-week-old broiler chickens (Hubbard-White Mountain Cross) were fed a standard broiler finishing ration containing 14.16 mg (14)C-avilamycin/kg diet (equivalent to 15 mg avilamycin activity/kg in the diet) for 4, 7 or 10 days. Medicated ration was provided ad libitum throughout the dosing phase. At the end of each dosing period, two birds of each sex were deprived of food and water for 6 h, and then samples of muscle, liver, abdominal fat, kidney and skin with subcutaneous fat were collected for radiochemical analysis. Radioactive residue levels in muscle and kidney were lower than the detection limits of 0.008 and 0.024 ug/g, respectively, at all sampling times. The mean peak level of 0.039 ug/g was attained in liver after 7 days of dosing. After 10 days of dosing, the mean total radioactive residues in skin, liver and fat, expressed as avilamycin equivalents, were 0.018, 0.022 and 0.024 ug/g, respectively. Steady-state concentrations of radioactivity were attained in all tissues within 4-7 days after the initiation of dosing.
WHO/FAO; Seventieth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 61: Avilamycin p.7 (2009). Available from, as of October 16, 2015: https://www.inchem.org/pages/jecfa.html
For more Absorption, Distribution and Excretion (Complete) data for AVILAMYCIN (6 total), please visit the HSDB record page.
Six rats (three males and three females) were fed a ration containing uniformly radiolabelled (14)C- avilamycin at a concentration of 550 mg/kg diet for 4.5 days. Urine and feces were collected during the dosing period, and livers were collected at zero withdrawal. Avilamycin A constituted approximately 19% of the fecal radioactivity. There were three major metabolites derived from the oligosaccharide and eurekanate portion of avilamycin in fecal samples. The most abundant metabolite in feces was flambic acid (metabolite B). Flambic acid was relatively unstable and readily converted to flambalactone (metabolite A).
WHO/FAO; Seventieth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 61: Avilamycin p.5 (2009). Available from, as of October 16, 2015: https://www.inchem.org/pages/jecfa.html
The minimum inhibitory concentrations (MIC) of eight antibiotics and two anticoccidial agents were determined for Clostridium perfringens strains isolated from 26 commercial broiler farms and 22 commercial turkey farms. Isolates were obtained from the intestines of birds on the farm or as the processing plant using standard culture and identification techniques. The microbroth dilution test was used to determine the MIC for each compound. Most isolates from chickens had MICs in the range of 2-16 mg/L for tilmicosin, tylosin and virginiamycin, whereas the MICs for avilamycin, avoparcin, monensin, narasin and penicillin were < or = 1 mg/L. Most strains from chickens had high MICs (> or = 64 mg/L) and appeared to be resistant to bacitracin and lincomycin. Most turkey isolates had MICs in the range of 2-16 mg/L for bacitracin, tilmicosin, tylosin and virginiamycin, with strains exhibiting MICs < or = 1 mg/L for avilamycin, avoparcin, monensin, narasin and penicillin. Several turkey isolates had MICs > or = 64 mg/L to lincomycin. No attempt was made to associate farm usage of a particular antibiotic to the antibiograms.
PMID:9057262 Watkins KL et al; Vet Microbiol 54 (2): 195-200 (1997)
... Nine crossbred pigs (five males and four females) weighing approximately 44 kg each were fed a ration containing 76.19 mg (14)C-avilamycin/kg in the diet (equivalent to 80 mg avilamycin activity/kg in the diet) at 12-hr intervals for 4, 7 or 10 days. ... One major metabolite observed in the extracts of both liver and excreta was flambic acid, which was formed as a result of cleavage of the ortho ester linking the C and D rings of avilamycin. Flambic acid represented 40-50% of the total radioactive residue in urine and feces and 15-20% of the residue in liver.
WHO/FAO; Seventieth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 61: Avilamycin p.6 (2009). Available from, as of October 16, 2015: https://www.inchem.org/pages/jecfa.html
Avilamycin is poorly absorbed and is extensively metabolized in the gut of pigs. Only about 8% of total radioactivity in pig feces was attributable to parent avilamycin. Metabolites were found in liver, whereas they were not detected in other tissues. The primary metabolite is flambic acid, representing 40-50% of the total radioactive residue in urine and feces and 15-20% of the residue in liver. No microbiologically active residues were detected in liver. Avilamycin is unlikely to be persistent in the environment following excretion from treated animals, as it is highly metabolized or degraded in animals.
WHO/FAO; Seventieth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 61: Avilamycin p.8 (2009). Available from, as of October 16, 2015: https://www.inchem.org/pages/jecfa.html
The antibiotic growth promoter avilamycin inhibits protein synthesis by binding to bacterial ribosomes. Here the binding site is further characterized on Escherichia coli ribosomes. The drug interacts with domain V of 23S rRNA, giving a chemical footprint at nucleotides A2482 and A2534. Selection of avilamycin-resistant Halobacterium halobium cells revealed mutations in helix 89 of 23S rRNA. Furthermore, mutations in helices 89 and 91, which have previously been shown to confer resistance to evernimicin, give cross-resistance to avilamycin. These data place the binding site of avilamycin on 23S rRNA close to the elbow of A-site tRNA. It is inferred that avilamycin interacts with the ribosomes at the ribosomal A-site interfering with initiation factor IF2 and tRNA binding in a manner similar to evernimicin.
PMID:1238433 Kofoed CB, Vester B; Antimicrob Agents Chemother 46 (11): 3339-42 (2002)
ABOUT THIS PAGE
93
PharmaCompass offers a list of Avilamycin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Avilamycin manufacturer or Avilamycin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Avilamycin manufacturer or Avilamycin supplier.
PharmaCompass also assists you with knowing the Avilamycin API Price utilized in the formulation of products. Avilamycin API Price is not always fixed or binding as the Avilamycin Price is obtained through a variety of data sources. The Avilamycin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Avilamycin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Avilamycin, including repackagers and relabelers. The FDA regulates Avilamycin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Avilamycin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Avilamycin manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Avilamycin supplier is an individual or a company that provides Avilamycin active pharmaceutical ingredient (API) or Avilamycin finished formulations upon request. The Avilamycin suppliers may include Avilamycin API manufacturers, exporters, distributors and traders.
click here to find a list of Avilamycin suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
Avilamycin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Avilamycin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Avilamycin GMP manufacturer or Avilamycin GMP API supplier for your needs.
A Avilamycin CoA (Certificate of Analysis) is a formal document that attests to Avilamycin's compliance with Avilamycin specifications and serves as a tool for batch-level quality control.
Avilamycin CoA mostly includes findings from lab analyses of a specific batch. For each Avilamycin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Avilamycin may be tested according to a variety of international standards, such as European Pharmacopoeia (Avilamycin EP), Avilamycin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Avilamycin USP).
