
1. Azelaic Acid, Dilithium Salt
2. Azelaic Acid, Dipotassium Salt
3. Azelaic Acid, Disodium Salt
4. Azelaic Acid, Monosodium Salt
5. Azelaic Acid, Potassium Salt
6. Azelaic Acid, Sodium Salt
7. Azelex
8. Finacea
9. Monosodium Azelate
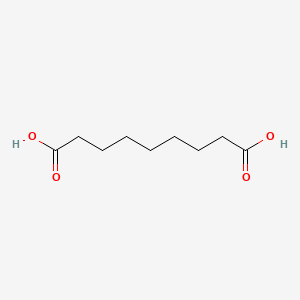
10. Nonanedioic Acid
11. Skinoren
1. Nonanedioic Acid
2. 123-99-9
3. Finacea
4. Anchoic Acid
5. Azelex
6. Lepargylic Acid
7. 1,7-heptanedicarboxylic Acid
8. Skinoren
9. 1,9-nonanedioic Acid
10. Heptanedicarboxylic Acid
11. N-nonanedioic Acid
12. Emerox 1110
13. Emerox 1144
14. Azelate
15. Acide Azelaique
16. Finevin
17. Azelainic Acid
18. Acidum Azelaicum
19. Poly(azelaic Anhydride)
20. Polyazelaic Anhydride
21. Skinorem
22. 1,7-dicarboxyheptane
23. Azelaic Acid, Technical Grade
24. Emery's L-110
25. Zk 62498
26. Zk-62498
27. Nonanedioic Acid, Homopolymer
28. 26776-28-3
29. Nsc 19493
30. Chebi:48131
31. Nsc-19493
32. Water-soluble Azelaic Acid
33. F2vw3d43yt
34. Mls000069659
35. Azelaate
36. Nsc19493
37. Mfcd00004432
38. Ncgc00014993-07
39. Smr000059164
40. Acido Azelaico
41. Azalaic Acid
42. Dsstox_cid_1640
43. Acide Azelaique [french]
44. Acido Azelaico [spanish]
45. Acidum Azelaicum [latin]
46. Dsstox_rid_76254
47. Dsstox_gsid_21640
48. Heptane-1,7-dicarboxylic Acid
49. Azelaic Acid [usan:inn]
50. Azelaic Polyanhydride
51. Azelaic
52. Azelaic Acid Polyanhydride
53. Cas-123-99-9
54. Finacea (tn)
55. Azelex (tn)
56. Sr-01000075671
57. Einecs 204-669-1
58. Unii-f2vw3d43yt
59. Azelaic Acid (usan/inn)
60. Brn 1101094
61. Azelaicacidtech
62. Azelainsaeure
63. Lepargylate
64. Nonandisaeure
65. Anchoate
66. Azelaic-acid
67. N-nonanedioate
68. Ai3-06299
69. Hsdb 7659
70. 1tuf
71. 1,9-nonanedioate
72. Sh-441
73. Agn-191861
74. Azelaic Acid, 98%
75. Spectrum_000057
76. Water-solubleazelaicacid
77. Opera_id_740
78. Polyazelaic Polyanhydride
79. 1,7-heptanedicarboxylate
80. Spectrum2_000995
81. Spectrum3_000278
82. Spectrum4_000401
83. Spectrum5_001304
84. C9-120-alpha-polymorph
85. C9-140-alpha-polymorph
86. C9-180-alpha-polymorph
87. C9-220-alpha-polymorph
88. C9-260-alpha-polymorph
89. C9-298-alpha-polymorph
90. Azelaic Acid [mi]
91. Epitope Id:187039
92. A-9800
93. Ec 204-669-1
94. Nonanedioic Acid Homopolymer
95. Azelaic Acid [inn]
96. Lopac-246379
97. Schembl3887
98. Azelaic Acid [hsdb]
99. Azelaic Acid [inci]
100. Azelaic Acid [usan]
101. Chembl1238
102. Lopac0_000051
103. Azelaic Acid [vandf]
104. Bspbio_001756
105. Kbiogr_000662
106. Kbioss_000437
107. Nonanedioic Acid Azelaic Acid
108. 4-02-00-02055 (beilstein Handbook Reference)
109. Mls001148615
110. Azelaic Acid [mart.]
111. Bidd:gt0315
112. Divk1c_000532
113. Spectrum1500648
114. Spbio_001089
115. Azelaic Acid [who-dd]
116. Gtpl7484
117. Dtxsid8021640
118. Hms501k14
119. Kbio1_000532
120. Kbio2_000437
121. Kbio2_003005
122. Kbio2_005573
123. Kbio3_001256
124. Azelaic Acid, Analytical Standard
125. Ninds_000532
126. Hms1921o11
127. Hms2092e22
128. Hms2234d10
129. Hms3260k03
130. Hms3372j07
131. Pharmakon1600-01500648
132. Azelaic Acid [orange Book]
133. Bcp18690
134. Hy-b0704
135. Zinc1531036
136. Tox21_110063
137. Tox21_201989
138. Tox21_303011
139. Tox21_500051
140. Azelaic Acid, Technical Grade, 80%
141. Ccg-40081
142. Lmfa01170054
143. Nsc757406
144. S4550
145. Stl059432
146. Akos000120052
147. Tox21_110063_1
148. Azelaic Acid, Technical, ~85% (gc)
149. Azelaic Acid, Vetec(tm) Reagent Grade
150. Db00548
151. Ks-5293
152. Lp00051
153. Nsc-757406
154. Sdccgmls-0066619.p001
155. Sdccgmls-0066619.p033
156. Sdccgsbi-0050040.p004
157. Idi1_000532
158. Mls-0066619
159. Ncgc00014993-01
160. Ncgc00014993-02
161. Ncgc00014993-03
162. Ncgc00014993-04
163. Ncgc00014993-05
164. Ncgc00014993-06
165. Ncgc00014993-08
166. Ncgc00014993-09
167. Ncgc00014993-10
168. Ncgc00014993-12
169. Ncgc00014993-15
170. Ncgc00093565-01
171. Ncgc00093565-02
172. Ncgc00093565-03
173. Ncgc00093565-04
174. Ncgc00093565-05
175. Ncgc00093565-06
176. Ncgc00093565-07
177. Ncgc00256508-01
178. Ncgc00259538-01
179. Ncgc00260736-01
180. Bp-27863
181. Mls-0066619.p021
182. Sbi-0050040.p003
183. A0561
184. Dicarboxylic Acid C9; Nonanedioic Acid; Aza
185. Eu-0100051
186. Ft-0626920
187. C08261
188. D03034
189. D70171
190. Ab00052140_12
191. Q413504
192. Sr-01000075671-1
193. Sr-01000075671-4
194. Sr-01000075671-6
195. 0c50d8ec-0db0-4f24-8efc-2919e1f0d9bf
196. Z57127532
197. F8889-5093
| Molecular Weight | 188.22 g/mol |
|---|---|
| Molecular Formula | C9H16O4 |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 8 |
| Exact Mass | 188.10485899 g/mol |
| Monoisotopic Mass | 188.10485899 g/mol |
| Topological Polar Surface Area | 74.6 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 147 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Azelex |
| PubMed Health | Azelaic Acid (On the skin) |
| Drug Classes | Antiacne Antibacterial |
| Drug Label | AZELEX (azelaic acid cream) 20% contains azelaic acid, a naturally occurring saturated dicarboxylic acid. Structural Formula: HOOC-(CH2)7-COOH Chemical Name: 1,7-heptanedicarboxylic acid Empirical Formula: C9H16O4Molecular Weight: 188.22... |
| Active Ingredient | Azelaic acid |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 20% |
| Market Status | Prescription |
| Company | Allergan |
| 2 of 4 | |
|---|---|
| Drug Name | Finacea |
| Drug Label | FINACEA (azelaic acid) Gel, 15%, contains azelaic acid, a naturally occurring saturated dicarboxylic acid. Chemically, azelaic acid is 1,7-heptanedicarboxylic acid, with the molecular formula C9 H16 O4, a molecular weight of 188.22, and the structu... |
| Active Ingredient | Azelaic acid |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 15% |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
| 3 of 4 | |
|---|---|
| Drug Name | Azelex |
| PubMed Health | Azelaic Acid (On the skin) |
| Drug Classes | Antiacne Antibacterial |
| Drug Label | AZELEX (azelaic acid cream) 20% contains azelaic acid, a naturally occurring saturated dicarboxylic acid. Structural Formula: HOOC-(CH2)7-COOH Chemical Name: 1,7-heptanedicarboxylic acid Empirical Formula: C9H16O4Molecular Weight: 188.22... |
| Active Ingredient | Azelaic acid |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 20% |
| Market Status | Prescription |
| Company | Allergan |
| 4 of 4 | |
|---|---|
| Drug Name | Finacea |
| Drug Label | FINACEA (azelaic acid) Gel, 15%, contains azelaic acid, a naturally occurring saturated dicarboxylic acid. Chemically, azelaic acid is 1,7-heptanedicarboxylic acid, with the molecular formula C9 H16 O4, a molecular weight of 188.22, and the structu... |
| Active Ingredient | Azelaic acid |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 15% |
| Market Status | Prescription |
| Company | Bayer Hlthcare |
Azelaic acid 20% cream is used topically in the treatment of mild to moderate inflammatory acne vulgaris. The drug is not indicated in the treatment of noninflammatory acne vulgaris. Therapy of acne vulgaris must be individualized and frequently modified depending on the types of acne lesions that predominate and the response to therapy. Results of several studies indicate that topical azelaic acid 20% cream is more effective than vehicle placebo in the treatment of mild to moderate inflammatory acne and as effective as topical tretinoin or benzoyl peroxide. Limited data indicate that topical azelaic acid also may be as effective as oral tetracycline hydrochloride in the management of acne vulgaris. A decrease in the number of inflammatory lesions occurs in most patients within 1-2 months of topical azelaic acid therapy, although maximum benefit generally requires more prolonged treatment.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 3601
Azelaic acid 15% gel is used topically for the treatment of inflammatory lesions (papules and pustules) associated with mild to moderate rosacea in adults. In 2 clinical studies in adults with mild to moderate papulopustular rosacea, therapy with azelaic acid 15% gel (applied twice daily for 12 weeks) resulted in a 50-58% reduction in the number of papules and pustules compared with a 38-40% reduction in patients receiving vehicle alone. Patients were instructed to avoid spicy foods, thermally hot foods and drinks, and alcoholic beverages during the treatment period, as well as to use only very mild soaps or soapless cleansing lotion for facial cleaning. Azelaic acid 20% cream also has been used with some success in the treatment of papulopustular rosacea. /Use is not currently included in the labeling approved by the US Food and Drug Administration/
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 3601
The physiopathologic mechanism of acne seems to be dependent on four main factors: a) sebum production and excretion; b) type of keratinization of the follicular channel; c) microbial colonization of the pilosebaceous unit and d) inflammatory reaction of the perifollicular area. Azelaic acid is effective in the treatment of acne because it possesses an activity against all of these factors. Azelaic acid is a competitive inhibitor of mitochondrial oxidoreductases and of 5 alpha-reductase, inhibiting the conversion of testosterone to 5-dehydrotestosterone. It also possesses bacteriostatic activity to both aerobic and anaerobic bacteria including Propionibacterium acnes. Azelaic acid is an anti-keratinizing agent, displaying antiproliferative cytostatic effects on keratinocytes and modulating the early and terminal phases of epidermal differentiation.
PMID:2534550 Passi S et al; G Ital Dermatol Venereol 24 (10): 455-63 (1989)
The effects of azelaic acid (AZA) on the epidermis of 47 individuals (12 with normal skin, 15 with seborrheic skin and 20 suffering from acne) and on in vitro cultured keratinocytes are reported. Topical application of a 20% AZA cream significantly improved the lesions of acne patients, but failed to induce clinically detectable changes in normal or seborrheic epidermis. Complementary investigations clearly showed that AZA treatment failed to induce specific changes in sebum composition, excretion rate, or in the size of sebaceous glands, but modified epidermal keratinization. Keratohyalin granules and tonofilament bundles were reduced in size and number, mitochondria were swollen and the rough endoplasmic reticulum of malpighian keratinocytes enlarged. The infundibular epidermis of acne individuals showed marked reduction of the horny layer thickness, widening of the horny cell cytoplasm, transitional corneal cells, normalization of filaggrin distribution, and the comedo contained few bacteria and spores. In vitro, AZA exerted marked time- and dose-dependent antiproliferative cytostatic effects on cultured keratinocytes, with a 50% inhibitory dose of 20 mM, decreased some keratinocyte proteins (highly soluble fractions S2, keratohyalin macroaggregate R2, and non-cross-linked fibrous protein S4) and a 95 kD and a 35 kD protein of the cytosolic fraction. Mitochondria were frequently damaged and the rough endoplasmic reticulum enlarged. Our results indicate that AZA is an antikeratinizing agent, displaying antiproliferative cytostatic effects on keratinocytes and modulating the early and terminal phases of epidermal differentiation.
PMID:2475995 Mayer-da-Silva A et al; Acta Derm Venereol Suppl (Stockh) 143: 20-30 (1989)
For more Therapeutic Uses (Complete) data for 1,7-HEPTANEDICARBOXYLIC ACID (7 total), please visit the HSDB record page.
There have been isolated reports of hypopigmentation after use of azelaic acid. Since azelaic acid has not been well studied in patients with dark complexions, these patients should be monitored for early signs of hypopigmentation.
US Natl Inst Health; DailyMed. Current Medication Information for Azelex (Azelaic Acid) Cream (May 2004). Available from, as of November 19, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3191#nlm34090-1
Topical therapy for rosacea aims to reduce inflammatory lesions and decrease erythema but can carry side effects such as stinging, pruritus, and burning. Metronidazole and azelaic acid gel 15% are U.S. Food and Drug Administration-approved for the treatment of rosacea. The current study was conducted to assess the cumulative irritation potential of 2 formulations of metronidazole 0.75% gel and 1% gel--and azelaic acid gel 15% over 21 days (N=36). Results of this study demonstrated a significantly greater potential for irritation from azelaic acid compared with metronidazole gel 0.75% (P<0.0001), which had significantly greater potential for irritation compared with metronidazole gel 1% (P=0.0054).
PMID:17500380 Colon LE et al; Cutis 79 (4): 317-21 (2007)
FDA Pregnancy Risk Category: B /NO EVIDENCE OF RISK IN HUMANS. Adequate, well controlled studies in pregnant women have not shown increased risk of fetal abnormalities despite adverse findings in animals, or, in the absence of adequate human studies, animal studies show no fetal risk. The chance of fetal harm is remote but remains a possibility./
US Natl Inst Health; DailyMed. Current Medication Information for Azelex (Azelaic Acid) Cream (May 2004). Available from, as of November 19, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3191#nlm34090-1
In patients using azelaic acid formulations, the following additional adverse experiences have been reported rarely: worsening of asthma, vitiligo depigmentation, small depigmented spots, hypertrichosis, reddening (signs of keratosis pilaris), and exacerbation of recurrent herpes labialis.
US Natl Inst Health; DailyMed. Current Medication Information for Azelex (Azelaic Acid) Cream (May 2004). Available from, as of November 19, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3191#nlm34090-1
For more Drug Warnings (Complete) data for 1,7-HEPTANEDICARBOXYLIC ACID (6 total), please visit the HSDB record page.
For the topical treatment of mild-to-moderate inflammatory acne vulgaris.
FDA Label
Azelaic acid is a saturated dicarboxylic acid found naturally in wheat, rye, and barley. It is a natural substance that is produced by Malassezia furfur (also known as Pityrosporum ovale), a yeast that lives on normal skin. It is effective against a number of skin conditions, such as mild to moderate acne, when applied topically in a cream formulation of 20%. It works in part by stopping the growth of skin bacteria that cause acne, and by keeping skin pores clear. Azelaic acid's antimicrobial action may be attributable to inhibition of microbial cellular protein synthesis.
Dermatologic Agents
Drugs used to treat or prevent skin disorders or for the routine care of skin. (See all compounds classified as Dermatologic Agents.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
D10AX03
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
D - Dermatologicals
D10 - Anti-acne preparations
D10A - Anti-acne preparations for topical use
D10AX - Other anti-acne preparations for topical use
D10AX03 - Azelaic acid
Absorption
Approximately 4% of the topically applied azelaic acid is systemically absorbed.
Route of Elimination
Azelaic acid is mainly excreted unchanged in the urine, but undergoes some -oxidation to shorter chain dicarboxylic acids.
...Azelaic acid (AA, C9 dicarboxylic acid)... when administered perorally to humans, at the same concentrations as the other /dicarboxylic acids/ (DA), it reaches much higher serum and urinary concentrations. Serum concentrations and urinary excretion obtained with intravenous or intra-arterial infusions of AA are significantly higher than those achievable by oral administration. Together with AA, variable amounts of its catabolites, mainly pimelic acid, are found in serum and urine, indicating an involvement of mitochondrial beta-oxidative enzymes. Short-lived serum levels of AA follow a single 1 hr intravenous infusion, but prolonging the period of infusion with successive doses of similar concentration produces sustained higher levels during the period of administration. These levels are consistent with the concentrations of AA capable of producing a cytotoxic effect on tumoral cells in vitro. AA is capable of crossing the blood-brain barrier: its concentration in the cerebrospinal fluid is normally in the range of 2-5% of the values in the serum.
PMID:2505463 Passi S et al; Acta Derm Venereol Suppl (Stockh) 143: 8-13 (1989)
Azelaic acid was the first dicarboxylic acid proposed as an alternative energy substrate in total parenteral nutrition. In this study, the pharmacokinetics of azelaic acid were investigated in 12 healthy volunteers, 7 receiving a constant infusion (10 g over 90 min) and 5 a bolus dose (1g). The 24 hr urinary excretion and plasma concentration in blood samples taken at regular intervals were assayed by gas-liquid chromatography. Experimental data were analysed by a 2-compartment nonlinear model that describes both tubular secretion and cellular uptake in Michaelis-Menten terms. A high value of urinary excretion (mean 76.9% of infused dose) and a mean clearance of 8.42 L/hr were found, suggesting the presence of tubular secretion. Estimating the population mean of the pharmacokinetic model parameters gave a maximal cellular uptake of 0.657 g/hr. The model predicts that 90% of the maximal uptake should be reached in the plateau phase of a constant infusion of 2.2 g/hr. The presence of extensive and rapid losses through urinary excretion, and the low estimated value of the maximal cellular uptake, indicate that azelaic acid is not suitable as an energy substrate for total parenteral nutrition.
PMID:1908756 Bertuzzi A et al; Clin Pharmacokinet 20 (5): 411-9 (1991)
Follicular concentrations of azelaic acid (AzA) were determined in vivo using a rapid, non-invasive method, after a single topical application of 20% (w/w) AzA cream, in order to establish whether the in vitro antimicrobial effects observed in previous studies are relevant in vivo. Preweighed amounts of 20% (w/w) AzA cream were applied over demarcated areas on the forehead and back of nine young adults, and samples were taken over a period of 5 hr. AzA was removed from the skin surface by washing with acetone, and follicular casts were collected using cyanacrylate gel. The samples were centrifuged to remove particulate matter, and the supernatants derivatized for analysis by HPLC. Although the results showed wide-ranging variability, the follicular concentration increased as the amount present on the surface declined. The maximum follicular concentrations of AzA attained ranged from 7.5 to 52.5 ng (micrograms of follicular casts)-1 and 0.5 to 23.4 ng/(ug of follicular casts) in samples taken from the back and forehead, respectively. Assuming an average density of follicular material of 0.9 g/mL, the mean maximum follicular concentration attained on the back was between 36 and 251 mmol/L, and on the forehead was between 2 and 112 mmol/L, and indicates that the concentration of AzA attained in follicular casts after a single topical application is comparable with the concentration required to inhibit the growth of Propionibacterium acnes and Staphylococcus epidermidis, in vitro.
PMID:8217752 Bojar RA et al; Br J Dermatol 129 (4): 399-402 (1993)
Six healthy male volunteers received a single topical treatment with 5 g of an anti-acne cream containing 20% azelaic acid (AzA) onto the face, the chest and the upper back. One week later 1 g of AzA was given orally to the same subjects as aqueous microcrystalline suspension. Following the two treatments the renal excretion of the unchanged compound was measured. Analysis included ether extraction of the urine, derivatization of extract and HPLC with UV detection. After topical application 2.2 +/- 0.7%, and after oral administration 61.2 +/- 8.8% of the dose had been excreted unchanged with the urine. By comparing both amounts, the percutaneous absorption of AzA from the cream was assessed to 3.6% of the dermally applied dose.
PMID:1365318 Tauber U et al; Exp Dermatol 1 (4): 176-9 (1992)
For more Absorption, Distribution and Excretion (Complete) data for 1,7-HEPTANEDICARBOXYLIC ACID (7 total), please visit the HSDB record page.
Mainly excreted unchanged in the urine but undergoes some b-oxidation to shorter chain dicarboxylic acids.
Approximately 60% of an oral-dose is excreted unchanged in the urine within 12 hr, and it is partly metabolized by -oxidation. After 8 hr, 6% of the radioactivity from a tracer dose of [14C]azelaic acid to rats was recovered as 14CO2. Successive cleavage by -oxidation results in the formation of pimelic and glutaric acids and subsequently malonyl-CoA and acetyl-CoA. Thus, azelaic acid is incorporated into fatty acid biosynthesis and the citric acid cycle
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 5:773
Pimelic acid is largely excreted unchanged in humans and dogs; the extent varies with the dose. Some degree of -oxidation occurs with dicarboxylic acids and, results in the formation of dicarboxylic acids that have two fewer carbon atoms than the parent acid. Pimelic acid has been identified as a metabolite of azelaic acid in microorganisms.
Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. 5:770
The observed half-lives in healthy subjects are approximately 45 minutes after oral dosing and 12 hours after topical dosing, indicating percutaneous absorption rate-limited kinetics.
The observed half-lives in healthy subjects are approximately 45 minutes after oral dosing and 12 hours after topical dosing,
US Natl Inst Health; DailyMed. Current Medication Information for Azelex (Azelaic Acid) Cream (May 2004). Available from, as of November 19, 2008: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=3191#nlm34090-1
The exact mechanism of action of azelaic acid is not known. It is thought that azelaic acid manifests its antibacterial effects by inhibiting the synthesis of cellular protein in anaerobic and aerobic bacteria, especially Staphylococcus epidermidis and Propionibacterium acnes. In aerobic bacteria, azelaic acid reversibly inhibits several oxidoreductive enzymes including tyrosinase, mitochondrial enzymes of the respiratory chain, thioredoxin reductase, 5-alpha-reductase, and DNA polymerases. In anaerobic bacteria, azelaic acid impedes glycolysis. Along with these actions, azelaic acid also improves acne vulgaris by normalizing the keratin process and decreasing microcomedo formation. Azelaic acid may be effective against both inflamed and noninflamed lesions. Specifically, azelaic acid reduces the thickness of the stratum corneum, shrinks keratohyalin granules by reducing the amount and distribution of filaggrin (a component of keratohyalin) in epidermal layers, and lowers the number of keratohyalin granules.
Azelaic acid, and other saturated dicarboxylic acids (C9-C12), are shown to be competitive inhibitors of tyrosinase (KI azelaic acid = 2.73 X 10(-3) M) and of membrane-associated thioredoxin reductase (KI azelaic acid = 1.25 X 10(-5) M). The monomethyl ester of azelaic acid does not inhibit thioredoxin reductase, but it does inhibit tyrosinase, although double the concentration is necessary compared with azelaic acid (KI azelaic acid monomethyl ester = 5.24 X 10(-3) M). Neither azelaic acid nor its monomethyl ester inhibit tyrosinase when catechol is used as a substrate instead of L-tyrosine. Therefore, the weak inhibitory action of azelaic acid on tyrosinase appears to be due to the competition of a single carboxylate group on this inhibitor for the alpha-carboxylate binding site of the L-tyrosine substrate on the enzyme active site. Based on the inhibitor constant on tyrosinase, at least cytotoxic levels of azelaic acid would be required for the direct inhibition of melanin biosynthesis in melanosomes if this mechanism is responsible for depigmentation in the hyperpigmentation disorders lentigo maligna and melasma. Alternatively only 10(-5) M azelaic acid is required to inhibit thioredoxin reductase. This enzyme is shown to regulate tyrosinase through a feedback mechanism involving electron transfer to intracellular thioredoxin, followed by a specific interaction between reduced thioredoxin and tyrosinase. Furthermore, the thioredoxin reductase/thioredoxin system is shown to be a principal electron donor for the ribonucleotide reductases which regulates DNA synthesis.
PMID:2114832 Schallreuter KU, Wood JW; Arch Dermatol Res 282 (3): 168-71 (1990)
The exact mechanism of action of topically applied azelaic acid in the treatment of acne vulgaris has not been fully elucidated; however, the effect appears to result partly from the antibacterial activity of the drug. Azelaic acid inhibits the growth of susceptible organisms (principally Propionibacterium acnes) on the surface of the skin by inhibiting protein synthesis. In addition, the drug also may inhibit follicular keratinization, which may prevent development or maintenance of comedones. Azelaic acid usually is bacteriostatic in action, but may be bactericidal in high concentrations against P. acnes and Staphylococcus epidermidis. Azelaic acid also exhibits antiproliferative effects against hyperactive and abnormal melanocytes but does not exhibit an appreciable depigmenting effect on normally pigmented skin.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 3601