
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Azadose
2. Azithromycin Dihydrate
3. Azithromycin Monohydrate
4. Azitrocin
5. Azythromycin
6. Cp 62993
7. Cp-62993
8. Cp62993
9. Dihydrate, Azithromycin
10. Goxal
11. Monohydrate, Azithromycin
12. Sumamed
13. Toraseptol
14. Ultreon
15. Vinzam
16. Zentavion
17. Zithromax
18. Zitromax
1. Zithromax
2. 83905-01-5
3. Sumamed
4. Hemomycin
5. Azasite
6. Zitromax
7. Zmax
8. Azithromycine
9. Azithromycinum
10. Azenil
11. Aziromycin
12. Zithromac
13. Azithromycine [french]
14. Azithromycinum [latin]
15. Azithromycin Dihydrate
16. Cp 62993
17. Cp-62993
18. Azitromax
19. Misultina
20. Tromix
21. Azithromycin Anhydrous
22. Azithrocin
23. Aruzilina
24. Macrozit
25. Trozocina
26. Xithrone
27. Zythromax
28. Aziwin
29. Durasite
30. Xz-450
31. Toraseptol
32. Anhydrous Azithromycin
33. 9-deoxo-9a-aza-9a-methyl-9a-homoerythromycin A
34. J2klz20u1m
35. Chebi:2955
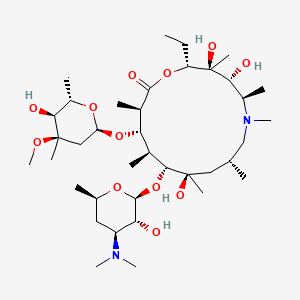
36. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-11-(((2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yl)oxy)-2-ethyl-3,4,10-trihydroxy-13-(((2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yl)oxy)-3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecan-15-one
37. Azitrocin
38. Xz 405
39. Xz 450
40. Nsc-758625
41. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-11-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-2-ethyl-3,4,10-trihydroxy-13-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecan-15-one
42. Ncgc00090753-01
43. Aritromicina
44. Mixoterin
45. Zithrax
46. Zitrotek
47. Aziwok
48. Aztrin
49. Setron
50. Zitrim
51. Tobil
52. Zifin
53. Cp-62,993
54. Zeto
55. Zmas
56. Zithromax Iv
57. Dsstox_cid_10760
58. Dsstox_rid_78858
59. Dsstox_gsid_30760
60. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-15-oxo-11-{[3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyl]oxy}-1-oxa-6-azacyclopentadecan-13-yl 2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranoside
61. Z-pak
62. C38h72n2o12
63. Aritromicina [spanish]
64. Azitromicine
65. Azithromycin (as Dihydrate)
66. Trulimax
67. Zentavion
68. Drg-0104
69. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-11-{[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy}-2-ethyl-3,4,10-trihydroxy-13-{[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy}-3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecan-15-one
70. 13-[(2,6-dideoxy-3-c-methyl-3-o-methyl-?-l-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-?-d-xylo-hexopyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one
71. Azm
72. Smr000471864
73. Zit
74. Azithromycin (zithromax)
75. Cas-83905-01-5
76. Ccris 1961
77. Azithromycin [inn]
78. Hsdb 7205
79. Z-pak (azithromycin)
80. Azithromycin (anhydrous)
81. Sr-05000002067
82. Azithromycin, Antibiotic For Culture Media Use Only
83. Unii-j2klz20u1m
84. Brn 5387583
85. Azithramycine
86. Azitromicina
87. Zithromycin
88. Azifast
89. Azigram
90. Azimakrol
91. Azitromin
92. Azyter
93. Azithromycin [usan:inn:ban]
94. Nsc643732
95. Azithromycin (aids Initiative)
96. Azithromycin,(s)
97. Zmax Sr
98. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-11-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-2-ethyl-3,4,10-trihydroxy-13-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,6
99. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-15-oxo-11-{[3,4,6-trideoxy-3-(dimethylamino)-b-d-xylo-hexopyranosyl]oxy}-1-oxa-6-azacyclopentadecan-13-yl 2,6-dideoxy-3-c-methyl-3-o-methyl-a-l-ribo-hexopyranoside
100. Zitromax Avium 600
101. Azitromicina [spanish]
102. Spectrum_000307
103. Azithromycin, Unspecified
104. Dch3
105. Spectrum2_001582
106. Spectrum3_000653
107. Spectrum4_000186
108. Spectrum5_001867
109. Azithromycin [mi]
110. Chembl529
111. Azithromycin [hsdb]
112. Ec 617-500-5
113. Schembl23481
114. Bspbio_002285
115. Kbiogr_000731
116. Kbioss_000787
117. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-13-((2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranosyl)oxy)-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-((3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyl)oxy)-1-oxa-6-azacyclopentadecan-15-one
118. 1-oxa-6-azacyclopentadecan-15-one, 13-((2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranosyl)oxy)-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-((3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyl)oxy)-, (2r-(2r*,3s*,4r*,5r*,8r*,10r*,11r*,12s*,13s*,14r*))-
119. Mls001055353
120. Mls001066331
121. Mls001201763
122. Mls001304005
123. Mls001332499
124. Mls001332500
125. Azithromycin [who-dd]
126. Azithromycin, Unspecified Form
127. Bidd:gt0792
128. Divk1c_000233
129. Spectrum1503679
130. N-methyl-11-aza-10-deoxo-10-dihydroerythromycin A
131. Spbio_001544
132. Gtpl6510
133. Dtxsid8030760
134. Azithromycin, Analytical Standard
135. Hms500l15
136. Kbio1_000233
137. Kbio2_000787
138. Kbio2_003355
139. Kbio2_005923
140. Kbio3_001505
141. Ninds_000233
142. Hms1922g12
143. Hms2094m11
144. Hms2232m10
145. Hms3259d10
146. Act03224
147. Azithromycin [usp Monograph]
148. Tox21_111008
149. Tox21_201011
150. Bdbm50373918
151. Ccg-39360
152. Mfcd00873574
153. Zinc85537026
154. Akos015895044
155. Db00207
156. Nc00712
157. Nsc 758625
158. Azithromycin 100 Microg/ml In Methanol
159. Idi1_000233
160. Ncgc00090753-02
161. Ncgc00090753-03
162. Ncgc00090753-04
163. Ncgc00090753-06
164. Ncgc00258564-01
165. Ac-16014
166. Cp62,993
167. Hy-17506
168. Sbi-0206706.p001
169. Cp-62993-3
170. A-9940
171. A-9941
172. N46072
173. Ab00698251-10
174. Ab00698251_11
175. 905a015
176. A905251
177. Q165399
178. Sr-05000002067-1
179. Sr-05000002067-2
180. Brd-k74501079-001-18-1
181. Z1563146014
182. Azithromycin, European Pharmacopoeia (ep) Reference Standard
183. Azithromycin Identity, United States Pharmacopeia (usp) Reference Standard
184. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-11-((2s,3r,4s,6r)-
185. Azithromycin For Peak Identification, European Pharmacopoeia (ep) Reference Standard
186. Azithromycin For System Suitability, European Pharmacopoeia (ep) Reference Standard
187. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-11-((2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyltetrahydro-2h-pyran-2-yloxy)-2-ethyl-3,4,10-trihydroxy-13-((2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2h-pyran-2-yloxy)-3,5,6,8,10,12,14-heptamethy...
188. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-11-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-2-ethyl-3,4,10-trihydroxy-13-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyl-tetrahydropyran-2-yl]oxy-3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecan-15-one
189. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)-11-[(2s,3r,4s,6r)-4-dimethylamino-3-hydroxy-6-methyloxan-2-yl]oxy-2-ethyl-3,4,10-trihydroxy-13-[(2r,4r,5s,6s)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy-3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecan-15-one
190. (2r,3s,4r,5r,8r,10r,11r,12s,13s,14r)13-((2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranosyl)oxy)-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-((3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyl)oxy)-1-oxa-6-azacyclopentadecan-15-one
191. [2r-(2r*,3s*,4r*,5r*,8r*,10r*,11r*,12s*,13s*,14r*)]-13-[(2,6-dideoxy-3-c-methyl-3-o-methyl-.alpha.-l-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-
192. 13-[(2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranosyl)oxy]-2-ethyl-3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyl]oxy]-1-oxa-6-azacyclopentadecan-15-one
| Molecular Weight | 749.0 g/mol |
|---|---|
| Molecular Formula | C38H72N2O12 |
| XLogP3 | 4 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 7 |
| Exact Mass | 748.50852574 g/mol |
| Monoisotopic Mass | 748.50852574 g/mol |
| Topological Polar Surface Area | 180 Ų |
| Heavy Atom Count | 52 |
| Formal Charge | 0 |
| Complexity | 1150 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 18 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 8 | |
|---|---|
| Drug Name | Azasite |
| Drug Label | AzaSite (azithromycin ophthalmic solution) is a 1% sterile aqueous topical ophthalmic solution of azithromycin formulated in DuraSite (polycarbophil, edetate disodium, sodium chloride). AzaSite is an off-white, viscous liquid with an osmolality of... |
| Active Ingredient | Azithromycin |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Oak Pharms Akorn |
| 2 of 8 | |
|---|---|
| Drug Name | Azithromycin |
| PubMed Health | Azithromycin (Into the eye) |
| Drug Classes | Antibiotic |
| Drug Label | Azithromycin tablets and azithromycin for oral suspension contain the active ingredient azithromycin, an azalide, a subclass of macrolide antibiotics, for oral administration. Azithromycin has the chemical name (2R,3S,4R,5R,8R, 10R,11R,12S,13S,14R)-1... |
| Active Ingredient | Azithromycin |
| Dosage Form | Injectable; Tablet; Solution; For suspension |
| Route | ophthalmic; Injection; Oral |
| Strength | 1%; eq 600mg base; eq 500mg base; eq 500mg base/vial; eq 100mg base/5ml; eq 250mg base; eq 200mg base/5ml |
| Market Status | Tentative Approval; Prescription |
| Company | Wockhardt; Sagent Strides; Teva Pharms; Fresenius Kabi Usa; Hospira; Gland Pharma; Teva; Apotex; Sun Pharm Inds; Pliva; Sandoz; Mylan |
| 3 of 8 | |
|---|---|
| Drug Name | Zithromax |
| PubMed Health | Azithromycin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | ZITHROMAX (azithromycin tablets and azithromycin for oral suspension) contain the active ingredient azithromycin, an azalide, a subclass of macrolide antibiotics, for oral administration. Azithromycin has the chemical name (2R,3S,4R,5R,8R, 10R,11R,12... |
| Active Ingredient | Azithromycin |
| Dosage Form | Tablet; Injectable; For suspension |
| Route | Injection; Oral |
| Strength | eq 500mg base; eq 100mg base/5ml; eq 250mg base; eq 1gm base/packet; eq 200mg base/5ml; eq 600mg base; eq 500mg base/vial |
| Market Status | Prescription |
| Company | Pfizer |
| 4 of 8 | |
|---|---|
| Drug Name | Zmax |
| PubMed Health | Azithromycin |
| Drug Classes | Antibiotic |
| Drug Label | Zmax (azithromycin extended release) for oral suspension contains the active ingredient azithromycin (as azithromycin dihydrate), an azalide, a subclass of macrolide antibiotics. Azithromycin has the chemical name (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)... |
| Active Ingredient | Azithromycin |
| Dosage Form | For suspension, extended release |
| Route | Oral |
| Strength | eq 2gm base/bot |
| Market Status | Prescription |
| Company | Pf Prism Cv |
| 5 of 8 | |
|---|---|
| Drug Name | Azasite |
| Drug Label | AzaSite (azithromycin ophthalmic solution) is a 1% sterile aqueous topical ophthalmic solution of azithromycin formulated in DuraSite (polycarbophil, edetate disodium, sodium chloride). AzaSite is an off-white, viscous liquid with an osmolality of... |
| Active Ingredient | Azithromycin |
| Dosage Form | Solution/drops |
| Route | Ophthalmic |
| Strength | 1% |
| Market Status | Prescription |
| Company | Oak Pharms Akorn |
| 6 of 8 | |
|---|---|
| Drug Name | Azithromycin |
| PubMed Health | Azithromycin (Into the eye) |
| Drug Classes | Antibiotic |
| Drug Label | Azithromycin tablets and azithromycin for oral suspension contain the active ingredient azithromycin, an azalide, a subclass of macrolide antibiotics, for oral administration. Azithromycin has the chemical name (2R,3S,4R,5R,8R, 10R,11R,12S,13S,14R)-1... |
| Active Ingredient | Azithromycin |
| Dosage Form | Injectable; Tablet; Solution; For suspension |
| Route | ophthalmic; Injection; Oral |
| Strength | 1%; eq 600mg base; eq 500mg base; eq 500mg base/vial; eq 100mg base/5ml; eq 250mg base; eq 200mg base/5ml |
| Market Status | Tentative Approval; Prescription |
| Company | Wockhardt; Sagent Strides; Teva Pharms; Fresenius Kabi Usa; Hospira; Gland Pharma; Teva; Apotex; Sun Pharm Inds; Pliva; Sandoz; Mylan |
| 7 of 8 | |
|---|---|
| Drug Name | Zithromax |
| PubMed Health | Azithromycin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | ZITHROMAX (azithromycin tablets and azithromycin for oral suspension) contain the active ingredient azithromycin, an azalide, a subclass of macrolide antibiotics, for oral administration. Azithromycin has the chemical name (2R,3S,4R,5R,8R, 10R,11R,12... |
| Active Ingredient | Azithromycin |
| Dosage Form | Tablet; Injectable; For suspension |
| Route | Injection; Oral |
| Strength | eq 500mg base; eq 100mg base/5ml; eq 250mg base; eq 1gm base/packet; eq 200mg base/5ml; eq 600mg base; eq 500mg base/vial |
| Market Status | Prescription |
| Company | Pfizer |
| 8 of 8 | |
|---|---|
| Drug Name | Zmax |
| PubMed Health | Azithromycin |
| Drug Classes | Antibiotic |
| Drug Label | Zmax (azithromycin extended release) for oral suspension contains the active ingredient azithromycin (as azithromycin dihydrate), an azalide, a subclass of macrolide antibiotics. Azithromycin has the chemical name (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)... |
| Active Ingredient | Azithromycin |
| Dosage Form | For suspension, extended release |
| Route | Oral |
| Strength | eq 2gm base/bot |
| Market Status | Prescription |
| Company | Pf Prism Cv |
Anti-Bacterial Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2012)
Azithromycin is used orally in children for the treatment of acute otitis media (AOM) caused by Haemophilus influenzae, M. catarrhalis, or S. pneumoniae. /Included in US product labeling/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 225
Azithromycin is used orally for the treatment of pharyngitis and tonsillitis caused by Streptococcus pyogenes (group A beta-hemolytic streptococci) in adults and children when first-line therapy (penicillins) cannot be used. /Included in US product labeling/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 225
Although further study is needed, azithromycin has been used in conjunction with an antimalarial agent (e.g., chloroquine, quinine, artesunate [not commercially available in the US]) for the treatment of uncomplicated malaria caused by Plasmodium falciparum, including multidrug-resistant strains. Azithromycin should not be used alone as monotherapy for the treatment of malaria. /NOT included in US product labeling/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 229
For more Therapeutic Uses (Complete) data for AZITHROMYCIN (52 total), please visit the HSDB record page.
Prolonged cardiac repolarization and QT interval, imparting a risk of developing cardiac arrhythmia and torsades de pointes, have been seen in treatment with macrolides, including azithromycin. Cases of torsades de pointes have been spontaneously reported during postmarketing surveillance in patients receiving azithromycin. Providers should consider the risk of QT prolongation which can be fatal when weighing the risks and benefits of azithromycin for at-risk groups including: patients with known prolongation of the QT interval, a history of torsades de pointes, congenital long QT syndrome, bradyarrhythmias or uncompensated heart failure; patients on drugs known to prolong the QT interval; or patients with ongoing proarrhythmic conditions such as uncorrected hypokalemia or hypomagnesemia, clinically significant bradycardia, and in patients receiving Class IA (quinidine, procainamide) or Class III (dofetilide, aminodarone, sotalol) antiarrhythmic agents. Elderly patients may be more susceptible to drug-associated effects on the QT interval.
US FDA; Label Information for ZITHROMAX (azithromycin tablets) and (azithromycin for oral suspension) (Last updated January 2013). Available from, as of March 15, 2013: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/050710s039,050711s036,050784s023lbl.pdf
Pregnancy risk category: B /NO EVIDENCE OF RISK IN HUMANS. Adequate, well controlled studies in pregnant women have not shown increased risk of fetal abnormalities despite adverse findings in animals, or, in the absents of adequate human studies, animal studies show no fetal risk. The chance of fetal harm is remote but remains a possibility./
Physicians Desk Reference. 58th ed. Thomson PDR. Montvale, NJ 2004., p. 2684
The most frequent adverse effects of azithromycin involve the GI tract (i.e., diarrhea/loose stools, nausea, abdominal pain). While these adverse effects generally are mild to moderate in severity and occur less frequently than with oral erythromycin, adverse GI effects are the most frequent reason for discontinuing azithromycin therapy. Administration of conventional azithromycin tablets or oral suspension with food may improve GI tolerability.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 234
Azithromycin has been detected in human milk. The drug should be used with caution in nursing women.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 237
For more Drug Warnings (Complete) data for AZITHROMYCIN (30 total), please visit the HSDB record page.
Azithromycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria in order to prevent the development antimicrobial resistance and maintain the efficacy of azithromycin. Azithromycin is indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the microorganisms listed in the specific conditions below. Recommended dosages, duration of therapy and considerations for various patient populations may vary among these infections. Refer to the FDA label and "Indications" section of this drug entry for detailed information. **Adults**: Acute bacterial exacerbations of chronic obstructive pulmonary disease due to _Haemophilus influenzae_, _Moraxella catarrhalis_ or _Streptococcus pneumoniae_ Acute bacterial sinusitis due to _Haemophilus influenzae_, _Moraxella catarrhalis_ or _Streptococcus pneumoniae_ Community-acquired pneumonia due to _Chlamydophila pneumoniae_, _Haemophilus influenzae_, _Mycoplasma pneumoniae_ or _Streptococcus pneumoniae_ in patients appropriate for oral therapy Pharyngitis/tonsillitis caused by _Streptococcus pyogenes_ as an alternative to first-line therapy in individuals who cannot use first-line therapy. Uncomplicated skin and skin structure infections due to _Staphylococcus aureus_, _Streptococcus pyogenes_, or _Streptococcus agalactiae_. Abscesses usually require surgical drainage. Urethritis and cervicitis due to _Chlamydia trachomatis_ or _Neisseria gonorrhoeae_. Genital ulcer disease in men due to _Haemophilus ducreyi_ (chancroid). Due to the small number of women included in clinical trials, the efficacy of azithromycin in the treatment of chancroid in women has not been established. **Pediatric Patients** Acute otitis media caused by _Haemophilus influenzae_, _Moraxella catarrhalis_ or _Streptococcus pneumoniae_ Community-acquired pneumonia due to _Chlamydophila pneumoniae_, _Haemophilus influenzae_, _Mycoplasma pneumoniae_ or _Streptococcus pneumoniae_ in patients appropriate for oral therapy. Pharyngitis/tonsillitis caused by _Streptococcus pyogenes_ as an alternative to first-line therapy in individuals who cannot use first-line therapy.
Treatment of bacterial conjunctivitis
Prevention of bronchopulmonary dysplasia
Macrolides stop bacterial growth by inhibiting protein synthesis and translation, treating bacterial infections. Azithromycin has additional immunomodulatory effects and has been used in chronic respiratory inflammatory diseases for this purpose.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01FA10
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01F - Macrolides, lincosamides and streptogramins
J01FA - Macrolides
J01FA10 - Azithromycin
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AA - Antibiotics
S01AA26 - Azithromycin
Absorption
Bioavailability of azithromycin is 37% following oral administration. Absorption is not affected by food. Macrolide absorption in the intestines is believed to be mediated by P-glycoprotein (ABCB1) efflux transporters, which are known to be encoded by the _ABCB1_ gene.
Route of Elimination
Biliary excretion of azithromycin, primarily as unchanged drug, is a major route of elimination. Over a 1 week period, approximately 6% of the administered dose is found as unchanged drug in urine.
Volume of Distribution
After oral administration, azithromycin is widely distributed in tissues with an apparent steady-state volume of distribution of 31.1 L/kg. Significantly greater azithromycin concentrations have been measured in the tissues rather than in plasma or serum,. The lung, tonsils and prostate are organs have shown a particularly high rate of azithromycin uptake. This drug is concentrated within macrophages and polymorphonucleocytes, allowing for effective activity against Chlamydia trachomatis. In addition, azithromycin is found to be concentrated in phagocytes and fibroblasts, shown by in vitro incubation techniques. In vivo studies demonstrate that concentration in phagocytes may contribute to azithromycin distribution to inflamed tissues.
Clearance
Mean apparent plasma cl=630 mL/min (following single 500 mg oral and i.v. dose)
Biliary excretion of azithromycin, predominantly as unchanged drug is a major route of elimination following oral administration.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 241
Azithromycin is rapidly absorbed from the GI tract after oral administration; absorption of the drug is incomplete but exceeds that of erythromycin. The absolute oral bioavailability of azithromycin is reported to be approximately 34-52% with single doses of 500 mg to 1.2 g administered as various oral dosage forms. Limited evidence indicates that the low bioavailability of zithromycin results from incomplete GI absorption rather acid degradation of the drug or extensive first-pss metabolism.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 240
Azithromycin appears to be distributed into most body tissues and fluids after oral or IV administration. The extensive tissue uptake of azithromycin has been attributed to cellular uptake of this basic antibiotic into relatively acidic lysosomes as a result of iron trapping and to an energy-dependent pathway associated with the nucleoside transport system.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 240
Because of rapid distribution into tissues and high intracellular concentrations of azithromycin, tissue concentrations of the drug generally exceed plasma concentrations by 10- to 100-fold following single dose administration; with multiple dosing, the tissue-to-plasma ratio increases.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 241
For more Absorption, Distribution and Excretion (Complete) data for AZITHROMYCIN (10 total), please visit the HSDB record page.
In vitro and in vivo studies to assess the metabolism of azithromycin have not been performed, however, this drug is eliminated by the liver,.
The principal route of biotransformation involves N-demethylation of the desosamine sugar or at the 9a position on the macrolide ring. Other metabolic pathways include O-demethylation and hydrolysis and/or hydroxylation of the cladinose and desosamine sugar moieties and the macrolide ring. Up to 10 metabolites of azithromycin have been identified, and all are microbiologically inactive. While short-term administration of azithromycin produces hepatic accumulation of the drug and increases azithromycin demethylase activity, current evidence indicates that hepatic cytochrome p450 induction of inactivation via cytochrome-metabolite complex formation does not occur. In contrast to erythromycin, azithromycin does not inhibit its own metabolism via this pathway.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 241
Terminal elimination half-life: 68 hours
An elimination half-life of 54.5 hours has been reported in children 4 months to 15 years of age receiving single or multiple oral doses of azithromycin.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 241
Plasma azithromycin concentrations following a single 500-mg oral or IV dose decline in a polyphasic manner with a terminal elimination half-life averaging 68 hours. The high values for apparent steady-state volume of distribution (31.3-33.3 L/kg) and plasma clearance (630 mL/minute, 10.18 mL/minute per kg) of azithromycin suggest that the prolonged half-life is related to extensive uptake and subsequent release of the drug from tissues. The average tissue half-life of azithromycin is estimated to be 1-4 days. The half-life of the drug in peripheral leukocytes ranges from 34-57 hours.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 241
In order to replicate, bacteria require a specific process of protein synthesis, enabled by ribosomal proteins. Azithromycin binds to the 23S rRNA of the bacterial 50S ribosomal subunit. It stops bacterial protein synthesis by inhibiting the transpeptidation/translocation step of protein synthesis and by inhibiting the assembly of the 50S ribosomal subunit,. This results in the control of various bacterial infections,. The strong affinity of macrolides, including azithromycin, for bacterial ribosomes, is consistent with their broadspectrum antibacterial activities. Azithromycin is highly stable at a low pH, giving it a longer serum half-life and increasing its concentrations in tissues compared to erythromycin.
Azithromycin usually is bacteriostatic, although the drug may be bactericidal in high concentrations against selected organisms. Bactericidal activity has been observed in vitro against Streptococcus pyogenes, S. pneumoniae, and Haemophilus influenzae. Azithromycin inhibits protein synthesis in susceptible organisms by penetrating the cell wall and binding to 50S ribosomal subunits, thereby inhibiting translocation of aminoacyl transfer-RNA and inhibiting polypeptide synthesis. The site of action of azithromycin appears to be the same as that of the macrolides (i.e., erythromycin, clarithromycin), clindamycin, lincomycin, and chloramphenicol. The antimicrobial activity of azithromycin is reduced at low pH. Azithromycin concentrates in phagocytes, including polymorphonuclear leukocytes, monocytes, macrophages, and fibroblasts. Penetration of the drug into phagocytic cells is necessary for activity against intracellular pathogens (e.g., Staphylococcus aureus, Legionella pneumophila, Chlamydia trachomatis, Salmonella typhi).
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 238

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
64
PharmaCompass offers a list of Azithromycin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Azithromycin manufacturer or Azithromycin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Azithromycin manufacturer or Azithromycin supplier.
PharmaCompass also assists you with knowing the Azithromycin API Price utilized in the formulation of products. Azithromycin API Price is not always fixed or binding as the Azithromycin Price is obtained through a variety of data sources. The Azithromycin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Azithromycin, unspecified form manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Azithromycin, unspecified form, including repackagers and relabelers. The FDA regulates Azithromycin, unspecified form manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Azithromycin, unspecified form API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Azithromycin, unspecified form manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Azithromycin, unspecified form supplier is an individual or a company that provides Azithromycin, unspecified form active pharmaceutical ingredient (API) or Azithromycin, unspecified form finished formulations upon request. The Azithromycin, unspecified form suppliers may include Azithromycin, unspecified form API manufacturers, exporters, distributors and traders.
click here to find a list of Azithromycin, unspecified form suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Azithromycin, unspecified form DMF (Drug Master File) is a document detailing the whole manufacturing process of Azithromycin, unspecified form active pharmaceutical ingredient (API) in detail. Different forms of Azithromycin, unspecified form DMFs exist exist since differing nations have different regulations, such as Azithromycin, unspecified form USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Azithromycin, unspecified form DMF submitted to regulatory agencies in the US is known as a USDMF. Azithromycin, unspecified form USDMF includes data on Azithromycin, unspecified form's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Azithromycin, unspecified form USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Azithromycin, unspecified form suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Azithromycin, unspecified form Drug Master File in Japan (Azithromycin, unspecified form JDMF) empowers Azithromycin, unspecified form API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Azithromycin, unspecified form JDMF during the approval evaluation for pharmaceutical products. At the time of Azithromycin, unspecified form JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Azithromycin, unspecified form suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Azithromycin, unspecified form Drug Master File in Korea (Azithromycin, unspecified form KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Azithromycin, unspecified form. The MFDS reviews the Azithromycin, unspecified form KDMF as part of the drug registration process and uses the information provided in the Azithromycin, unspecified form KDMF to evaluate the safety and efficacy of the drug.
After submitting a Azithromycin, unspecified form KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Azithromycin, unspecified form API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Azithromycin, unspecified form suppliers with KDMF on PharmaCompass.
A Azithromycin, unspecified form CEP of the European Pharmacopoeia monograph is often referred to as a Azithromycin, unspecified form Certificate of Suitability (COS). The purpose of a Azithromycin, unspecified form CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Azithromycin, unspecified form EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Azithromycin, unspecified form to their clients by showing that a Azithromycin, unspecified form CEP has been issued for it. The manufacturer submits a Azithromycin, unspecified form CEP (COS) as part of the market authorization procedure, and it takes on the role of a Azithromycin, unspecified form CEP holder for the record. Additionally, the data presented in the Azithromycin, unspecified form CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Azithromycin, unspecified form DMF.
A Azithromycin, unspecified form CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Azithromycin, unspecified form CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Azithromycin, unspecified form suppliers with CEP (COS) on PharmaCompass.
A Azithromycin, unspecified form written confirmation (Azithromycin, unspecified form WC) is an official document issued by a regulatory agency to a Azithromycin, unspecified form manufacturer, verifying that the manufacturing facility of a Azithromycin, unspecified form active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Azithromycin, unspecified form APIs or Azithromycin, unspecified form finished pharmaceutical products to another nation, regulatory agencies frequently require a Azithromycin, unspecified form WC (written confirmation) as part of the regulatory process.
click here to find a list of Azithromycin, unspecified form suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Azithromycin, unspecified form as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Azithromycin, unspecified form API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Azithromycin, unspecified form as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Azithromycin, unspecified form and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Azithromycin, unspecified form NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Azithromycin, unspecified form suppliers with NDC on PharmaCompass.
Azithromycin, unspecified form Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Azithromycin, unspecified form GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Azithromycin, unspecified form GMP manufacturer or Azithromycin, unspecified form GMP API supplier for your needs.
A Azithromycin, unspecified form CoA (Certificate of Analysis) is a formal document that attests to Azithromycin, unspecified form's compliance with Azithromycin, unspecified form specifications and serves as a tool for batch-level quality control.
Azithromycin, unspecified form CoA mostly includes findings from lab analyses of a specific batch. For each Azithromycin, unspecified form CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Azithromycin, unspecified form may be tested according to a variety of international standards, such as European Pharmacopoeia (Azithromycin, unspecified form EP), Azithromycin, unspecified form JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Azithromycin, unspecified form USP).
