
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
VMF
0
Australia

Annual Reports
NA
0
Weekly News Recap #Phispers
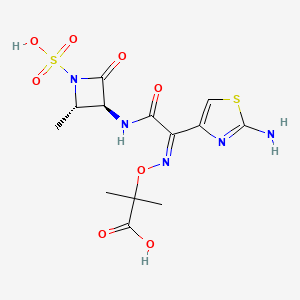

1. Az Threonam
2. Az-threonam
3. Azactam
4. Azthreonam
5. Sq 26,776
6. Sq-26,776
7. Sq26,776
8. Urobactam
1. Azactam
2. 78110-38-0
3. Primbactam
4. Azthreonam
5. Nebactam
6. Azonam
7. Aztreon
8. Rel-aztreonam
9. Sq 26776
10. Sq-26776
11. Nsc646279
12. Chembl158
13. Nsc-646279
14. 149496-40-2
15. Aztreonam E-isomer
16. Nsc-758913
17. 2-[(z)-[1-(2-amino-1,3-thiazol-4-yl)-2-[[(2s,3s)-2-methyl-4-oxo-1-sulfoazetidin-3-yl]amino]-2-oxoethylidene]amino]oxy-2-methylpropanoic Acid
18. Mfcd00072145
19. G2b4ve5gh8
20. Chebi:161680
21. Squibb 26776
22. Sr-01000841814
23. 2-[[(z)-[1-(2-amino-4-thiazolyl)-2-[[(2s,3s)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]amino]-2-oxoethylidene]amino]oxy]-2-methylpropionic Acid
24. (z)-2-((((2-amino-4-thiazolyl)(((2s,3s)-2-methyl-4-oxo-1-sulfo-3-azetidinyl)carbamoyl)methylene)amino)oxy)-2-methylpropionic Acid
25. Aztreonam (azactam, Cayston)
26. Azetreonam
27. E-aztreonam
28. Prestwick_914
29. Aztreonam [inn]
30. Aztreonam [jan]
31. Aztreonam [mi]
32. Aztreonam [usan]
33. Prestwick2_000185
34. Prestwick3_000185
35. Aztreonam [vandf]
36. Aztreonam [mart.]
37. Aztreonam [usp-rs]
38. Aztreonam [who-dd]
39. Bspbio_000109
40. [2s-[2alpha,3beta(z)]]-2-[[[1-(2-amino-4-thiazolyl)-2-[(2-methyl-4-oxo-1-sulfo-3-azetidinyl)amino]-2-oxoethylidene]amino]oxy]-2-methylpropanoic Acid
41. Mls003915628
42. Mls006011974
43. Aztreonam, Analytical Standard
44. Bidd:gt0765
45. Bpbio1_000121
46. Aztreonam [orange Book]
47. Dtxsid0022640
48. Aztreonam [usp Monograph]
49. Bcpp000356
50. Hms1568f11
51. Hms2090k09
52. Hms2095f11
53. Hms3712f11
54. Hy-b0129
55. Bdbm50240480
56. Zinc12503091
57. Akos015840157
58. Akos015961777
59. Ac-4330
60. Bcp9000372
61. Ccg-220185
62. Cs-1902
63. Ncgc00179656-01
64. 2-({[(1z)-1-(2-amino-1,3-thiazol-4-yl)-2-{[(2s,3s)-2-methyl-4-oxo-1-sulfoazetidin-3-yl]amino}-2-oxoethylidene]amino}oxy)-2-methylpropanoic Acid
65. 2-[(z)-[1-(2-aminothiazol-4-yl)-2-[[(2s,3s)-2-methyl-4-oxo-1-sulfo-azetidin-3-yl]amino]-2-oxo-ethylidene]amino]oxy-2-methyl-propanoic Acid
66. As-13760
67. Smr002204030
68. Smr004703537
69. So 26776
70. S1505
71. 110a380
72. Sr-01000841814-2
73. Sr-01000841814-3
74. Brd-k62607865-001-03-0
75. Q27262730
76. Aztreonam, United States Pharmacopeia (usp) Reference Standard
77. Aztreonam, Pharmaceutical Secondary Standard; Certified Reference Material
78. (2s,3s)-3-({(2z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-[(1-carboxy-1-methylethoxy)imino]ethanoyl}amino)-2-methyl-4-oxoazetidine-1-sulfonate
79. (2s,3s)-3-({(2z)-2-(2-ammonio-1,3-thiazol-4-yl)-2-[(1-carboxy-1-methylethoxy)imino]ethanoyl}amino)-2-methyl-4-oxoazetidine-1-sulfonate(aztreonam)
80. 2-((((e)-1-(2-aminothiazol-4-yl)-2-(((2s,3s)-2-methyl-4-oxo-1-sulfoazetidin-3-yl)amino)-2-oxoethylidene)amino)oxy)-2-methylpropanoic Acid
81. 2-((((z)-1-(2-aminothiazol-4-yl)-2-(((2s,3s)-2-methyl-4-oxo-1-sulfoazetidin-3-yl)amino)-2-oxoethylidene)amino)oxy)-2-methylpropanoic Acid
82. 2-[(z)-[1-(2-amino-1,3-thiazol-4-yl)-2-[[(2s)-2-methyl-4-oxo-1-sulfoazetidin-3-yl]amino]-2-oxoethylidene]amino]oxy-2-methylpropanoic Acid
83. 2-[1-(2-amino-thiazol-4-yl)-1-((2s,3s)-2-methyl-4-oxo-1-sulfo-azetidin-3-ylcarbamoyl)-meth-(z)-ylideneaminooxy]-2-methyl-propionic Acid
84. 2-aminothiazol-4-yl)-2-((2s,3s)-2-methyl-4-oxo-1-sulfoazetidin-3-ylamino)-2-oxoethylideneaminooxy)-2-methylpropanoic Acid
85. 80951-91-3
86. Propanoic Acid, 2-(((1-(2-amino-4-thiazolyl)-2-((2-methyl-4-oxo-1-sulfo-3-azetidinyl)amino)-2-oxoethylidene)amino)oxy)-2-methyl-, (2s-(2.alpha.,3.beta.(z)))-
87. Propanoic Acid, 2-[[(z)-[1-(2-amino-4-thiazolyl)-2-[[(2s,3s)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]amino]-2-oxoethylidene]amino]oxy]-2-methyl-
| Molecular Weight | 435.4 g/mol |
|---|---|
| Molecular Formula | C13H17N5O8S2 |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 7 |
| Exact Mass | 435.05185486 g/mol |
| Monoisotopic Mass | 435.05185486 g/mol |
| Topological Polar Surface Area | 238 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 808 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Azactam |
| PubMed Health | Aztreonam (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | AZACTAM (aztreonam injection) contains the active ingredient aztreonam, a monobactam. It was originally isolated from Chromobacterium violaceum. It is a synthetic bactericidal antibiotic.The monobactams, having a unique monocyclic beta-lactam nucle... |
| Active Ingredient | Aztreonam |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2gm/vial; 1gm/vial |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 2 of 6 | |
|---|---|
| Drug Name | Aztreonam |
| PubMed Health | Aztreonam |
| Drug Classes | Antibiotic |
| Drug Label | AZACTAM (aztreonam injection) contains the active ingredient aztreonam, a monobactam. It was originally isolated from Chromobacterium violaceum. It is a synthetic bactericidal antibiotic.The monobactams, having a unique monocyclic beta-lactam nucle... |
| Active Ingredient | Aztreonam |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/vial; 2gm/vial; 1gm/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Eurohlth Intl |
| 3 of 6 | |
|---|---|
| Drug Name | Cayston |
| PubMed Health | Aztreonam |
| Drug Classes | Antibiotic |
| Active Ingredient | Aztreonam |
| Dosage Form | For solution |
| Route | Inhalation |
| Strength | 75mg/vial |
| Market Status | Prescription |
| Company | Gilead |
| 4 of 6 | |
|---|---|
| Drug Name | Azactam |
| PubMed Health | Aztreonam (Injection) |
| Drug Classes | Antibiotic |
| Drug Label | AZACTAM (aztreonam injection) contains the active ingredient aztreonam, a monobactam. It was originally isolated from Chromobacterium violaceum. It is a synthetic bactericidal antibiotic.The monobactams, having a unique monocyclic beta-lactam nucle... |
| Active Ingredient | Aztreonam |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2gm/vial; 1gm/vial |
| Market Status | Prescription |
| Company | Bristol Myers Squibb |
| 5 of 6 | |
|---|---|
| Drug Name | Aztreonam |
| PubMed Health | Aztreonam |
| Drug Classes | Antibiotic |
| Drug Label | AZACTAM (aztreonam injection) contains the active ingredient aztreonam, a monobactam. It was originally isolated from Chromobacterium violaceum. It is a synthetic bactericidal antibiotic.The monobactams, having a unique monocyclic beta-lactam nucle... |
| Active Ingredient | Aztreonam |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 500mg/vial; 2gm/vial; 1gm/vial |
| Market Status | Prescription |
| Company | Fresenius Kabi Usa; Eurohlth Intl |
| 6 of 6 | |
|---|---|
| Drug Name | Cayston |
| PubMed Health | Aztreonam |
| Drug Classes | Antibiotic |
| Active Ingredient | Aztreonam |
| Dosage Form | For solution |
| Route | Inhalation |
| Strength | 75mg/vial |
| Market Status | Prescription |
| Company | Gilead |
Cayston is indicated for the suppressive therapy of chronic pulmonary infections due to Pseudomonas aeruginosa in patients with cystic fibrosis (CF) aged 6 years and older.
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Treatment of Gram-negative endobronchial infection in bronchiectasis patients
Treatment of Pseudomonas aeruginosa pulmonary infection / colonisation in patients with cystic fibrosis
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01DF01
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01D - Other beta-lactam antibacterials
J01DF - Monobactams
J01DF01 - Aztreonam
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : Complete
Rev. Date : 2012-12-24
Pay. Date : 2012-12-03
DMF Number : 19342
Submission : 2006-04-11
Status : Active
Type : II
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 16751
Submission : 2003-08-08
Status : Inactive
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2014-07-15
Pay. Date : 2013-10-30
DMF Number : 26856
Submission : 2013-10-18
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 17184
Submission : 2004-02-23
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38055
Submission : 2023-05-12
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2019-08-13
Pay. Date : 2019-06-03
DMF Number : 33762
Submission : 2019-04-09
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2019-07-09
Pay. Date : 2019-05-17
DMF Number : 33731
Submission : 2019-04-05
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 20907
Submission : 2007-09-07
Status : Inactive
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2014-05-28
Pay. Date : 2013-09-30
DMF Number : 27508
Submission : 2013-09-11
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23971
Submission : 2010-07-06
Status : Inactive
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registration Number : 219MF10225
Registrant's Address : VIA MURILLO, 7, 04013-SERMONETA (LATINA), ITALY
Initial Date of Registration : 2007-07-06
Latest Date of Registration : 2007-07-06

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registrant Name : Phamchem Service Co., Ltd.
Registration Date : 2025-02-25
Registration Number : 20250225-210-J-1786
Manufacturer Name : Chongqing Tiandi Pharmaceuti...
Manufacturer Address : No.1 Shenyang Road, Zhongzhou Avenue, Zhongxian county, Chongqing, China

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]NDC Package Code : 59651-859
Start Marketing Date : 2024-01-12
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (50kg/50kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 13538-001
Start Marketing Date : 2009-07-15
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (200kg/200kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 13538-009
Start Marketing Date : 2020-07-01
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (200kg/200kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 13538-008
Start Marketing Date : 2020-10-31
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (200kg/200kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 52952-001
Start Marketing Date : 2012-07-25
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 24525-0025
Start Marketing Date : 2015-12-14
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT FOR HUMAN P...

NDC Package Code : 54087-160
Start Marketing Date : 2012-01-04
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER, FOR SOLUTION (75mg/1)
Marketing Category : DRUG FOR FURTHER PROCESSING

NDC Package Code : 63592-0180
Start Marketing Date : 2017-11-21
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (100kg/100kg)
Marketing Category : BULK INGREDIENT

NDC Package Code : 61126-710
Start Marketing Date : 2023-08-03
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]About the Company : Transo-Pharm, a fully licensed and certified distributor, specializes in pharmaceutical components for the health and veterinary industries. It offers support to clients throughout...
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
About the Company : Founded in 1935, TAPI Technology & API Services has a long-standing tradition of advancing health through innovation and dedication. Today, we proudly build upon this legacy, drivi...
About the Company : Asia Pioneer Pharmaceuticals is a leading supplier of fine chemicals, pharmaceutical intermediates, active pharmaceutical ingredients and other compounds. It also offers finished p...

About the Company : Beijing Lunarsun pharma is a professional manufacturer of API and intermediates. The factory is approved by US FDA. According to EU and US FDA's requirement, we supply following AP...

About the Company : Curia is a global contract research, development and manufacturing organization (CDMO), offering products and services across the drug development spectrum to help our partners tur...

About the Company : Fuan Pharmaceutical (Group) Co., Ltd. was established on February 25, 2004. The company is located in Chongqing Changshou Economic and Technological Development Zone, covering an a...

About the Company : Globofarm International Pvt Ltd is a well-informed, highly efficient, Swiftly functioning competitive sourcing entity. We serve private, institutional, and corporate clients worldw...

About the Company : Hainan Haiyao is a national enterprise of high technology that integrates the research, development, production and sales of traditional Chinese medicine, antibiotic and oncology p...

About the Company : Hanways Chempharm Co., Limited, the former one is Hubei Hanways Pharchem CO.,Limited, set up in 2009 in Wuhan, China. We are engaged in supplying APIs, pharmaceutical intermediate...

About the Company : Teva was established in 1901. Our global headquarters are based in Israel. Today we have a portfolio of more than 3,500 medicines, and produce approximately 120 billion tablets and...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Details:
ADVANZ strengthens its specialty and hospital business by gaining an anti-infective portfolio from Sanofi made up of eight anti-infectives including Azactam (aztreonam), Cefotax, Claforan, Oroken, Rulid, Suprax, Wintriaxone and Colistimethate Sodique products.
Lead Product(s): Aztreonam
Therapeutic Area: Infections and Infectious Diseases Brand Name: Azactam
Study Phase: Approved FDFProduct Type: Antibiotic
Sponsor: Advanz Pharma
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Acquisition March 10, 2022
Lead Product(s) : Aztreonam
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Advanz Pharma
Deal Size : Undisclosed
Deal Type : Acquisition
Details : ADVANZ strengthens its specialty and hospital business by gaining an anti-infective portfolio from Sanofi made up of eight anti-infectives including Azactam (aztreonam), Cefotax, Claforan, Oroken, Rulid, Suprax, Wintriaxone and Colistimethate Sodique pro...
Product Name : Azactam
Product Type : Antibiotic
Upfront Cash : Undisclosed
March 10, 2022
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Emblaveo (aztreonam-avibactam), a beta-lactamase inhibitor, has been FDA approved for the treatment of adult patients with complicated intra-abdominal infections in patients 18 years and older.
Lead Product(s): Aztreonam,Avibactam,Metronidazole
Therapeutic Area: Infections and Infectious Diseases Brand Name: Emblaveo
Study Phase: Approved FDFProduct Type: Antibiotic
Sponsor: Pfizer Inc
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable February 07, 2025
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aztreonam,Avibactam,Metronidazole
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Pfizer Inc
Deal Size : Inapplicable
Deal Type : Inapplicable
U.S. FDA Approves EMBLAVEO™ for Complicated Intra-Abdominal Infections
Details : Emblaveo (aztreonam-avibactam), a beta-lactamase inhibitor, has been FDA approved for the treatment of adult patients with complicated intra-abdominal infections in patients 18 years and older.
Product Name : Emblaveo
Product Type : Antibiotic
Upfront Cash : Inapplicable
February 07, 2025
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Emblaveo (aztreonam-avibactam), a beta-lactamase inhibitor, is FDA approved for treating adult patients with complicated intra-abdominal infections, HAP, and cUTIs, including pyelonephritis.
Lead Product(s): Aztreonam,Avibactam,Metronidazole
Therapeutic Area: Infections and Infectious Diseases Brand Name: Emblaveo
Study Phase: Approved FDFProduct Type: Antibiotic
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable April 22, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aztreonam,Avibactam,Metronidazole
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
European Commission Approves Pfizer’s EMBLAVEO® for Resistant Infections
Details : Emblaveo (aztreonam-avibactam), a beta-lactamase inhibitor, is FDA approved for treating adult patients with complicated intra-abdominal infections, HAP, and cUTIs, including pyelonephritis.
Product Name : Emblaveo
Product Type : Antibiotic
Upfront Cash : Inapplicable
April 22, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
ATM-AVI (aztreonam-avibactam) is an investigational treatment for infections caused by Gram-negative bacteria with limited treatment options. It combines aztreonam, a monobactam β-lactam, with avibactam, a recent broad-spectrum β-lactamase inhibitor.
Lead Product(s): Aztreonam,Avibactam,Metronidazole
Therapeutic Area: Infections and Infectious Diseases Brand Name: Emblaveo
Study Phase: Phase IIIProduct Type: Antibiotic
Sponsor: Inapplicable
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 06, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Aztreonam,Avibactam,Metronidazole
Therapeutic Area : Infections and Infectious Diseases
Highest Development Status : Phase III
Partner/Sponsor/Collaborator : Inapplicable
Deal Size : Inapplicable
Deal Type : Inapplicable
Phase 3 Studies of Pfizer’s Novel Antibiotic Combination Offer New Treatment Hope for Patients w...
Details : ATM-AVI (aztreonam-avibactam) is an investigational treatment for infections caused by Gram-negative bacteria with limited treatment options. It combines aztreonam, a monobactam β-lactam, with avibactam, a recent broad-spectrum β-lactamase inhibitor.
Product Name : Emblaveo
Product Type : Antibiotic
Upfront Cash : Inapplicable
January 06, 2023

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 250MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : AZACTAM IN PLASTIC CONTAINER
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 40MG/ML
Packaging :
Approval Date : 1989-05-24
Application Number : 50632
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Azactam
Dosage Form : POWDER FOR INJECTION / INFUSION
Dosage Strength : -
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Azactam
Dosage Form : Powder for injection/infusion fluid, resolution
Dosage Strength : 2 g
Packaging : Hood glass
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : Injection
Dosage Strength : 2G
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
Brand Name : Cayston
Dosage Form : POWDER FOR NEBULISER SOLUTION
Dosage Strength : 75 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name :
Dosage Form : Vial
Dosage Strength : 2G
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : AZTREONAM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 2GM/VIAL
Packaging :
Approval Date : 2011-03-23
Application Number : 65286
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : AZTREONAM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 2GM/VIAL
Packaging :
Approval Date : 2021-11-08
Application Number : 206517
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : India
Brand Name :
Dosage Form : Powder for Injection
Dosage Strength : 1G
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

 USP reference standards; highly characterized specimens of drug substances, impurities, excipients, degradation products & more.
USP reference standards; highly characterized specimens of drug substances, impurities, excipients, degradation products & more.
CAS Number : 87500-74-1
Quantity Per Vial :
Price ($) : 805
Catalog Number : 1046409
Current Lot : R014U0
Previous Lot : H1K400 (29-FEB-2016)
NDC Code :

 USP reference standards; highly characterized specimens of drug substances, impurities, excipients, degradation products & more.
USP reference standards; highly characterized specimens of drug substances, impurities, excipients, degradation products & more.
CAS Number : N/A
Quantity Per Vial :
Price ($) : 805
Catalog Number : 1046307
Current Lot : R05390
Previous Lot : G1L370 (30-SEP-2017)
NDC Code :

 USP reference standards; highly characterized specimens of drug substances, impurities, excipients, degradation products & more.
USP reference standards; highly characterized specimens of drug substances, impurities, excipients, degradation products & more.
CAS Number : 78110-38-0
Quantity Per Vial :
Price ($) : 230
Catalog Number : 1046205
Current Lot : R08590
Previous Lot : R041F0 (30-SEP-2018)
NDC Code :

 USP reference standards; highly characterized specimens of drug substances, impurities, excipients, degradation products & more.
USP reference standards; highly characterized specimens of drug substances, impurities, excipients, degradation products & more.
CAS Number : 78110-38-0
Quantity Per Vial : 200
Sale Unit : mg
Price : $245.00
Details : Material Origin- Fermentation-Microbial; USMC...
Monograph :
Storage :
Code/Batch No : Catalog #1046205 / R08590

 USP reference standards; highly characterized specimens of drug substances, impurities, excipients, degradation products & more.
USP reference standards; highly characterized specimens of drug substances, impurities, excipients, degradation products & more.
CAS Number :
Quantity Per Vial : 15
Sale Unit : mg
Price : $919.00
Details : Material Origin- Chemical Synthesis; USMCA- N...
Monograph :
Storage :
Code/Batch No : Catalog #1046307 / R05390

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
27
PharmaCompass offers a list of Aztreonam API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Aztreonam manufacturer or Aztreonam supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Aztreonam manufacturer or Aztreonam supplier.
PharmaCompass also assists you with knowing the Aztreonam API Price utilized in the formulation of products. Aztreonam API Price is not always fixed or binding as the Aztreonam Price is obtained through a variety of data sources. The Aztreonam Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Aztreonam manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Aztreonam, including repackagers and relabelers. The FDA regulates Aztreonam manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Aztreonam API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Aztreonam manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Aztreonam supplier is an individual or a company that provides Aztreonam active pharmaceutical ingredient (API) or Aztreonam finished formulations upon request. The Aztreonam suppliers may include Aztreonam API manufacturers, exporters, distributors and traders.
click here to find a list of Aztreonam suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Aztreonam DMF (Drug Master File) is a document detailing the whole manufacturing process of Aztreonam active pharmaceutical ingredient (API) in detail. Different forms of Aztreonam DMFs exist exist since differing nations have different regulations, such as Aztreonam USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Aztreonam DMF submitted to regulatory agencies in the US is known as a USDMF. Aztreonam USDMF includes data on Aztreonam's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Aztreonam USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Aztreonam suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Aztreonam Drug Master File in Japan (Aztreonam JDMF) empowers Aztreonam API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Aztreonam JDMF during the approval evaluation for pharmaceutical products. At the time of Aztreonam JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Aztreonam suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Aztreonam Drug Master File in Korea (Aztreonam KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Aztreonam. The MFDS reviews the Aztreonam KDMF as part of the drug registration process and uses the information provided in the Aztreonam KDMF to evaluate the safety and efficacy of the drug.
After submitting a Aztreonam KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Aztreonam API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Aztreonam suppliers with KDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Aztreonam as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Aztreonam API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Aztreonam as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Aztreonam and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Aztreonam NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Aztreonam suppliers with NDC on PharmaCompass.
Aztreonam Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Aztreonam GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Aztreonam GMP manufacturer or Aztreonam GMP API supplier for your needs.
A Aztreonam CoA (Certificate of Analysis) is a formal document that attests to Aztreonam's compliance with Aztreonam specifications and serves as a tool for batch-level quality control.
Aztreonam CoA mostly includes findings from lab analyses of a specific batch. For each Aztreonam CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Aztreonam may be tested according to a variety of international standards, such as European Pharmacopoeia (Aztreonam EP), Aztreonam JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Aztreonam USP).
