

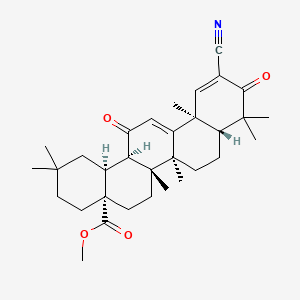

1. 2-cyano-3,12-dioxoolean-1,9-dien-28-oic Acid Methyl Ester
2. Cddo Methyl Ester
3. Cddo-me
4. Methyl 2-cyano-3,12-dioxoolean-1,9-dien-28-oate
5. Rta-402
1. 218600-53-4
2. Cddo-me
3. Cddo Methyl Ester
4. Rta 402
5. Nsc 713200
6. Cddo-methyl Ester
7. Rta-402
8. Nsc713200
9. Rta402
10. Methyl (4as,6ar,6bs,8ar,12as,14ar,14bs)-11-cyano-2,2,6a,6b,9,9,12a-heptamethyl-10,14-dioxo-1,3,4,5,6,7,8,8a,14a,14b-decahydropicene-4a-carboxylate
11. Tp-155
12. Ceg1q6ogu1
13. Bardoxolone Methyl [usan]
14. Nsc-713200
15. Methyl 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oate
16. 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic Acid Methyl Ester
17. Methyl (4as,6ar,6bs,12as,14ar,14br)-11-cyano-2,2,6a,6b,9,9,12a-heptamethyl-10,14-dioxo-1,3,4,5,6,7,8,8a,14a,14b-decahydropicene-4a-carboxylate
18. Bardoxolone-methyl
19. (4as,6ar,6bs,8ar,12as,14ar,14bs)-methyl 11-cyano-2,2,6a,6b,9,9,12a-heptamethyl-10,14-dioxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,12a,14,14a,14b-octadecahydropicene-4a-carboxylate
20. Mfcd11983137
21. Ncgc00186460-01
22. Bardoxolone (methyl)
23. Bardoxolone Methyl Ester
24. Unii-ceg1q6ogu1
25. Cddo-me, 2
26. Dsstox_cid_28690
27. Dsstox_rid_82960
28. Dsstox_gsid_48764
29. Methyl 2-cyano-3,12-dioxoolean-1,9-dien-28-oate
30. Gtpl3443
31. Bardoxolone Methyl (jan/usan)
32. Bardoxolone Methyl [jan]
33. Chembl1762621
34. Dtxsid5048764
35. Schembl12521530
36. Chebi:177406
37. 2-cyano-3,12-dioxoolean-1,9-dien-28-oic Acid Methyl Ester
38. 2-cyano-3,12-dioxooleana-1,9-dien-28-oic Acid Methyl Ester
39. Bdbm217379
40. Bardoxolone Methyl [who-dd]
41. Bcp04660
42. Zinc3982151
43. Tox21_113229
44. S8078
45. Bardoxolone Methyl Ester [mi]
46. Akos025401880
47. Ccg-269743
48. Cs-0598
49. Db05983
50. Cddo Methyl Ester, >=98% (hplc)
51. Ac-26830
52. Csa:218600-53-4;bardoxolone Methyl
53. Hy-13324
54. Cas-218600-53-4
55. D09585
56. Nsc 713200; Rta 402; Cddo Methyl Ester
57. A847580
58. Q4860208
59. (+)-methyl 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oate
60. (4as,6ar,6bs,8ar,12as,14bs)-methyl 11-cyano-2,2,6a,6b,9,9,12a-he
61. Oleana-1,9(11)-dien-28-oic Acid, 2-cyano-3,12-dioxo-, Methyl Ester
| Molecular Weight | 505.7 g/mol |
|---|---|
| Molecular Formula | C32H43NO4 |
| XLogP3 | 6.7 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 2 |
| Exact Mass | 505.31920885 g/mol |
| Monoisotopic Mass | 505.31920885 g/mol |
| Topological Polar Surface Area | 84.2 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 1210 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in lymphoma (unspecified), multiple myeloma, and solid tumors.
Treatment of Alport syndrome
RTA 402 inhibits the activity of nuclear factor kappa-B (NF-kB) activated by tumor necrosis factor (TNF) and other inflammatory agents in a variety of cancer cells. It is a novel targeted cancer therapy with a unique mechanism of action. It exploits fundamental physiological differences between cancerous and noncancerous cells by modulating oxidative stress response pathways. As a result, the drug is toxic to cancer cells but induces protective antioxidant and anti-inflammatory responses in normal cells.