
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
VMF
0
EDQM
0
USP
0
JP
0
Others
DRUG PRODUCT COMPOSITIONS
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
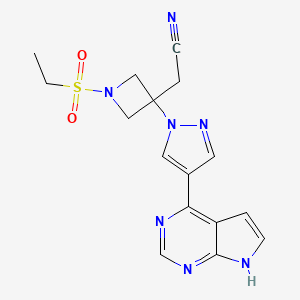

1. 3-azetidineacetonitrile, 1-(ethylsulfonyl)-3-(4-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-1h-pyrazol-1-yl)-
2. 3-azetidineacetonitrile, 1-(ethylsulfonyl)-3-(4-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-1h-pyrazol-1-yl)-, Phosphate (1:1)
3. Baricitinib Phosphate
4. Baricitinib Phosphate Salt
5. Incb-028050
6. Incb-28050
7. Incb028050
8. Ly-3009104
9. Ly3009104
10. Olumiant
1. 1187594-09-7
2. Olumiant
3. Incb028050
4. Ly3009104
5. 2-(3-(4-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-1h-pyrazol-1-yl)-1-(ethylsulfonyl)azetidin-3-yl)acetonitrile
6. Incb 028050
7. Incb-028050
8. Ly-3009104
9. 1-(ethylsulfonyl)-3-[4-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-1h-pyrazol-1-yl]-3-azetidineacetonitrile
10. Baricitinib (ly3009104, Incb028050)
11. 2-[1-ethylsulfonyl-3-[4-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]azetidin-3-yl]acetonitrile
12. Isp4442i3y
13. Ly 3009104
14. 3-azetidineacetonitrile, 1-(ethylsulfonyl)-3-(4-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-1h-pyrazol-1-yl)-
15. Ly3009104 (phosphate);incb028050 (phosphate)
16. Incb28050
17. Baricitinib [usan]
18. Baricitinib [usan:inn]
19. Unii-isp4442i3y
20. Incb 28050
21. Olumiant (tn)
22. 3-azetidineacetonitrile, 1-(ethylsulfonyl)-3-[4-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-1h-pyrazol-1-yl]-
23. 3jw
24. Baricitinib [mi]
25. Baricitinib [inn]
26. Baricitinib [jan]
27. Baricitinib [who-dd]
28. Mls006011247
29. Schembl871150
30. Baricitinib (jan/usan/inn)
31. Baricitinib (ly3009104)
32. Baricitinib [ema Epar]
33. Baricitinib (incb028050)
34. Gtpl7792
35. Chembl2105759
36. Ammd00005
37. Chebi:95341
38. Baricitinib [orange Book]
39. Dtxsid30152228
40. Ex-a413
41. Hms3651l17
42. Hms3672m15
43. Hms3747g21
44. Bcp04686
45. Bdbm50021656
46. Mfcd21608464
47. Nsc799357
48. S2851
49. Zinc73069247
50. Akos022186127
51. Akos025401933
52. Am81232
53. Bcp9000380
54. Ccg-268312
55. Cs-0724
56. Db11817
57. Ds-7641
58. Nsc-799357
59. Pb27275
60. Sb10845
61. Ncgc00345839-01
62. Ncgc00345839-14
63. Ncgc00345839-16
64. 2-(3-(4-(3h-pyrrolo[2,3-d]pyrimidin-4-yl)-1h-pyrazol-1-yl)-1-(ethylsulfonyl)azetidin-3-yl)acetonitrile
65. Ac-27404
66. Hy-15315
67. Smr004703006
68. Bcp0726000031
69. Baricitinib (incb28050, Ly3009104)
70. Ft-0775037
71. Sw220096-1
72. D10308
73. A892931
74. J-503551
75. Q4860707
76. (1-(ethylsulfonyl)-3-(4-(7h-pyrrolo(2,3-d)pyrimidin-4-yl)-1h-pyrazol-1-yl)azetidin-3-yl)ethanenitrile
77. {1-(ethylsulfonyl)-3-[4-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-1h-pyrazol-1-yl]azetidin-3-yl}acetonitrile
78. Incb 28050; Incb28050; Ly-3009104;1-(ethylsulfonyl)-3-[4-(7h-pyrrolo[2,3-d]pyrimidin-4-yl)-1h-pyrazol-1-yl]-3-azetidineacetonitrile
| Molecular Weight | 371.4 g/mol |
|---|---|
| Molecular Formula | C16H17N7O2S |
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 5 |
| Exact Mass | 371.11644398 g/mol |
| Monoisotopic Mass | 371.11644398 g/mol |
| Topological Polar Surface Area | 129 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 678 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for the treatment of moderate to severe active rheumatoid arthritis in adult patients who have responded inadequately to, or who are intolerant to one or more disease-modifying anti-rheumatic drugs as monotherapy or in combination with methotrexate.
FDA Label
Rheumatoid arthritis
Baricitinib is indicated for the treatment of moderate to severe active rheumatoid arthritis in adult patients who have responded inadequately to, or who are intolerant to one or more disease modifying anti rheumatic drugs. Olumiant may be used as monotherapy or in combination with methotrexate.
Atopic Dermatitis
Baricitinib is indicated for the treatment of moderate to severe atopic dermatitis in adult patients who are candidates for systemic therapy.
Alopecia areata
Baricitinib is indicated for the treatment of severe alopecia areata in adult patients (see section 5. 1).
Treatment of chronic idiopathic arthritis (including rheumatoid arthritis , ankylosing spondylarthritis , psoriatic arthritis and juvenile idiopathic arthritis )
Treatment of Systemic Lupus Erythematosus (SLE)
Treatment of Coronavirus disease 2019
Treatment of alopecia areata
Treatment of atopic dermatitis
L04AA37
L04AA37
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AA - Selective immunosuppressants
L04AA37 - Baricitinib
Absorption
Baricitinib diaplays a dose-proportional increase in systemic exposure in the therapeutic dose range with linear pharmacokinetics. When orally administered, baricitinb is rapidly absorbed with an oral bioavailability of approximately 79 % (CV = 3.94 %). It has a median time to reach peak plasma concentration (Tmax) of 1hour (range: 0.5-3hours). Food consumption affects the exposure by decreasing it by up to 14 %, and decreasing the peak plasma concentration (Cmax) by up to 18 % and Tmax by 0.5 hours.
Route of Elimination
In a clinical pharmacology study, baricitinib was excreted predominately as the unchanged active substance in urine (69 %) and feces (15 %) and only 4 minor oxidative metabolites were identified (3 in urine; 1 in feces) constituting approximately 5 % and 1 % of the dose, respectively.
Volume of Distribution
Mean volume of distribution following intravenous infusion administration is 76 L.
Clearance
Mean apparent clearance (CL/F) in patients with rheumatoid arthritis is approximately 9.42 L/hr (CV = 34.3 %).
Baricitinib undergoes oxidation by CYP3A4, although less than 10% of the total dose is prone to this biotransformation. There is no formation of quantifiable metabolites in the plasma.
Mean half-life in patients with rheumatoid arthritis is approximately 12.5 hrs (CV = 27.4 %).
JAK enzymes are part of the family of tyrosine kinases that constitutively bind to the intracellular domains of cytokine receptors and promote the signalling cascades of cytokines and growth factors involved in haematopoiesis, inflammation and immune function that are also implicated in the pathogenesis of rheumatoid arthritis. Circulating proinflammatory cytokines bind to these cell surface receptors. Upon binding of extracellular cytokines and growth factors, JAKs are phosphorylated and activate signal transducers and activators of transcription (STATs). Through the signalling cascades, inflammatory cytokine and chemokine transcription is induced to form inflammatory mediators including IL-2, IL-6, IL-12, IL-15, IL-23, IFN- and GM-CSF. Baricitinib selectively and reversibly inhibits JAK1 and JAK2 to modulates their signalling pathways, thereby reducing the phosphorylation and activation of STATs. In isolated enzyme assays, baricitinib also exhibited an inhibitory effect on other types of JAK enzymes,Tyrosine Kinase 2 and JAK3, at higher concentrations needed for JAK1/2 inhibition.
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
82
PharmaCompass offers a list of Baricitinib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Baricitinib manufacturer or Baricitinib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Baricitinib manufacturer or Baricitinib supplier.
PharmaCompass also assists you with knowing the Baricitinib API Price utilized in the formulation of products. Baricitinib API Price is not always fixed or binding as the Baricitinib Price is obtained through a variety of data sources. The Baricitinib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Baricitinib manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Baricitinib, including repackagers and relabelers. The FDA regulates Baricitinib manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Baricitinib API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Baricitinib manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Baricitinib supplier is an individual or a company that provides Baricitinib active pharmaceutical ingredient (API) or Baricitinib finished formulations upon request. The Baricitinib suppliers may include Baricitinib API manufacturers, exporters, distributors and traders.
click here to find a list of Baricitinib suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Baricitinib DMF (Drug Master File) is a document detailing the whole manufacturing process of Baricitinib active pharmaceutical ingredient (API) in detail. Different forms of Baricitinib DMFs exist exist since differing nations have different regulations, such as Baricitinib USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Baricitinib DMF submitted to regulatory agencies in the US is known as a USDMF. Baricitinib USDMF includes data on Baricitinib's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Baricitinib USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Baricitinib suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Baricitinib Drug Master File in Korea (Baricitinib KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Baricitinib. The MFDS reviews the Baricitinib KDMF as part of the drug registration process and uses the information provided in the Baricitinib KDMF to evaluate the safety and efficacy of the drug.
After submitting a Baricitinib KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Baricitinib API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Baricitinib suppliers with KDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Baricitinib as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Baricitinib API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Baricitinib as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Baricitinib and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Baricitinib NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Baricitinib suppliers with NDC on PharmaCompass.
Baricitinib Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Baricitinib GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Baricitinib GMP manufacturer or Baricitinib GMP API supplier for your needs.
A Baricitinib CoA (Certificate of Analysis) is a formal document that attests to Baricitinib's compliance with Baricitinib specifications and serves as a tool for batch-level quality control.
Baricitinib CoA mostly includes findings from lab analyses of a specific batch. For each Baricitinib CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Baricitinib may be tested according to a variety of international standards, such as European Pharmacopoeia (Baricitinib EP), Baricitinib JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Baricitinib USP).
