
Synopsis
Synopsis
0
USDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Canada

0
Australia

0
Listed Dossiers
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
API
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Barite
2. Baritop
3. Barium Sulfate (2:1)
4. E Z Cat
5. E-z-cat
6. Ezcat
7. Micropaque Oral
8. Sulfate, Barium
1. 7727-43-7
2. Barite
3. Baritop
4. Barytes
5. Barium Sulphate
6. Barite (ba(so4))
7. Barosperse
8. Barotrast
9. Esophotrast
10. Liquipake
11. Macropaque
12. Micropaque
13. Microtrast
14. Radiobaryt
15. Radiopaque
16. Entrobar
17. Enemark
18. Enecat
19. Blanc Fixe
20. Enamel White
21. 13462-86-7
22. Readi-cat
23. Liquid E-z-paque
24. E-z-paque
25. Pigment White 22
26. Baryte
27. C.i. Pigment White 21
28. E-z-hd
29. Barium(2+);sulfate
30. Sulfuric Acid, Barium Salt (1:1)
31. Baso4
32. Barimite-xf
33. Volumen
34. Spezialsorte Ai
35. Ci 77120
36. Tagitol V
37. Readi-cat2
38. Readi-cat2 Smoothies
39. C.i. Pigment White 22
40. Sachtoperse Ab-tm 18383
41. 25bb7eke2e
42. Mfcd00003455
43. Colonatrast
44. Liquibarine
45. Actybaryte
46. Bakontal
47. Baraflave
48. Baricon
49. Baridol
50. Barobag
51. Barocat
52. Barodense
53. Baroloid
54. Barytgen
55. Baryxine
56. Basofor
57. Bayrites
58. Citobaryum
59. Danobaryt
60. Finemeal
61. Intropaque
62. Lactobaryt
63. Microbar
64. Microfanox
65. Mixobar
66. Neobalgin
67. Novopaque
68. Oesobar
69. Oratrast
70. Polibar
71. Supramike
72. Suspobar
73. Tixobar
74. Tonopaque
75. Topcontral
76. Unibaryt
77. Eweiss
78. Neobar
79. Raybar
80. Travad
81. Rugar
82. Barii Sulphas
83. Prepcat
84. Baryx Colloidal
85. Barium Andreu
86. Eneset
87. Gastropaque-s
88. Hitone
89. Liquid Polibar
90. Radimix Colon
91. Baritogen Deluxe
92. Baryta White
93. Mikabarium B
94. Mikabarium F
95. Recto Barium
96. Barosperse Ii
97. Micropaque Rd
98. Radio-baryx
99. Sparkle Granules
100. Umbrasol A
101. Artificial Barite
102. Baritop P
103. Barium Sulfuricum
104. Redi-flow
105. Bar-test
106. Gel-unix
107. Liquid Barosperse
108. Unit-pak
109. Mixture Iii
110. Baritop G Powder
111. E-z Preparations
112. Esopho-cat
113. Ultra-r
114. Veri-o-pake
115. X-opac
116. Barosperse 110
117. Sol-o-pake
118. Tomocat Concentrate
119. Barytes 22
120. Epi-c
121. Liquid Polibar Plus
122. Liquid Sol-o-pake
123. Artificial Heavy Spar
124. Baritop 100
125. Baryum (sulfate De)
126. E-z-cat Concentrate
127. Readi-cat 2
128. Barium 100
129. Epi-stat 57
130. Epi-stat 61
131. Bf 10 (sulfate)
132. Barium Sulfate [jan]
133. Caswell No. 071b
134. Ci Pigment White 21
135. Tonojug 2000
136. Barium Sulphate, Natural
137. Barii Sulfas
138. Barium Sulfate (1:1)
139. Barium Sulfate (baso4)
140. Hd 200 Plus
141. E-z-ac
142. E-z-paste Esophageal Cream
143. Esophotrast Esophageal Cream
144. Precipitated Barium Sulphate
145. Tomocat 1000 Concentrate
146. Pigment White 21
147. Barium(2+) Sulfate
148. Hsdb 5041
149. Ba147
150. Hd 85
151. Ss 50
152. Einecs 231-784-4
153. Unii-25bb7eke2e
154. Epa Pesticide Chemical Code 007502
155. Barium Sulfate [usp:jan]
156. Varibar Nectar
157. Ai3-03611
158. Barite Powder
159. Barium- Sulphate
160. C.i. 77120
161. Baricon (tn)
162. Einecs 236-664-5
163. Varibar
164. Barium Sulfate Powder
165. Barium Sulfate, Powder
166. Readi-cat 2 Smoothies
167. Ec 231-784-4
168. Barium Sulfate, Precipitated
169. Barium Sulfate, Puratronic?
170. Barium Sulfate [ii]
171. Barium Sulfate [mi]
172. Barium Sulphate Nanoparticles
173. Barium Sulfate (jp17/usp)
174. Barium Sulfate [hsdb]
175. Barium Sulfate [inci]
176. Barium Sulfate [vandf]
177. Barium Sulfate [mart.]
178. Barium Sulfate [who-dd]
179. Barium Sulfate [who-ip]
180. Chembl2105897
181. Dtxsid0050471
182. Chebi:133326
183. Barium Sulfate Powder, 99% Nano
184. Ci 77120 [inci]
185. Barii Sulfas [who-ip Latin]
186. Barium Sulfate [orange Book]
187. Barium Sulfate [ep Monograph]
188. Akos015902784
189. Barium Sulfate [usp Monograph]
190. Db11150
191. Barium Sulfate Powder, 98% (4-5?m)
192. Barium Sulfate Nanoparticles / Nanopowder
193. Ft-0622575
194. Ft-0697585
195. Readi-cat 2 Component Barium Sulfate
196. Barium Sulfate Component Of Readi-cat 2
197. D02052
198. Q309038
199. Sr-01000944372
200. Sr-01000944372-1
201. 8054-35-1
| Molecular Weight | 233.39 g/mol |
|---|---|
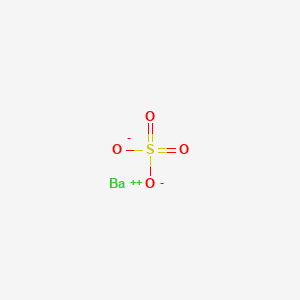
| Molecular Formula | BaO4S |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 233.856977 g/mol |
| Monoisotopic Mass | 233.856977 g/mol |
| Topological Polar Surface Area | 88.6 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 62.2 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
A compound used as an x-ray contrast medium that occurs in nature as the mineral barite.
National Library of Medicine's Medical Subject Headings online file (MeSH, 2011). Available from, as of November 11, 2010: https://www.nlm.nih.gov/cgi/mesh/2011/MB_cgi?term=Barium%20sulfate
Barium sulfate suspension is indicated for use as a contrast medium in x-ray diagnosis of the gastrointestinal tract. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for BAROSPERSE (barium sulfate) powder, for suspension (August 2010). Available from, as of November 12, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31506
This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA.
US Natl Inst Health; DailyMed. Current Medication Information for BAROSPERSE (barium sulfate) powder, for suspension (August 2010). Available from, as of November 12, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31506
Oral or rectal barium sulfate suspension, in low concentration, is indicated for enhancement of computed tomographic images (CT of the body) to delineate the gastrointestinal tract. /Included in US product labeling/
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 521
For more Therapeutic Uses (Complete) data for Barium sulfate (18 total), please visit the HSDB record page.
/VET/ Unless intestinal tract evacuates itself promptly, large masses of drug can produce an impaction. Peritoneal leakage has been associated with peritonitis, possibly secondary to ... fecal leakage. Use approved grades ... impure forms may be associated with metaplasia at ulcer sites in dogs.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 38
Rarely, oral barium sulfate suspensions may cause constipation or intestinal obstruction due to impaction in the colon. Cramping from distention of the intestine by barium sulfate suspensions or diarrhea may also occur. Retention of barium sulfate in the appendix has reportedly caused appendicitis. Barium sulfate fecaliths reported can lead to obstruction, intussusception, ulceration, or even intestinal perforation and may rarely require surgical removal. ... Aspiration of large amt may cause pneumonitis or nodular granulomas of interstitial lung tissues and lymph nodes; asphyxiation and death have occurred in one patient.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2189
Users must be certain that barium sulfate is not contaminated by sol barium salts. A convenient test is to shake up a portion with water and, to the clear supernatant portion, add a small amt of a soln of magnesium sulfate in water. Appearance of a precipitate indicates the presence of a sol barium salt.
Dreisbach, R.H. Handbook of Poisoning. 12th ed. Norwalk, CT: Appleton and Lange, 1987., p. 119
Barium sulfate products are contraindicated in patients with known or suspected obstruction of the colon, known or suspected gastrointestinal tract perforation, suspected tracheoesophageal fistula, obstructing lesions of the small intestine, pyloric stenosis inflammation or neoplastic lesions of the rectum, recent rectal biopsy, or known hypersensitivity to barium sulfate formulations.
US Natl Inst Health; DailyMed. Current Medication Information for BAROSPERSE (barium sulfate) powder, for suspension (August 2010). Available from, as of November 12, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=31506
For more Drug Warnings (Complete) data for Barium sulfate (49 total), please visit the HSDB record page.
Barium sulfate is a radiographic contrast agent indicated for use in computed tomography (CT) of the abdomen to delineate the gastrointestinal (GI) tract in adult and pediatric patients.
FDA Label
Barium sulfate increases the absorption of x-rays as they are passed throughout the body, delineating body structures, in which barium sulfate is localized. This allows for the clear visualization of normal organs/defect in normal anatomy.
Contrast Media
Substances used to allow enhanced visualization of tissues. (See all compounds classified as Contrast Media.)
Absorption
Barium sulfate is not absorbed following oral or rectal administration with a normal gastrointestinal tract. In patients with a normal GI tract, barium sulfate is normally excreted within 24 hr after oral ingestion. Post rectal administration of barium sulfate suspensions, the drug is generally excreted when the enema is released. Some barium may remain in the colon for several weeks, however, and eventually clears, especially in patients with impaired intestinal peristalsis. It is difficult to quantify the uptake of ingested barium because of a number of factors affect its absorption. The presence of sulfate in food can cause the precipitation of barium sulfate. The following is the approximate time to peak opacification of organs by barium sulfate in a healthy GI tract: Esophagus, stomach, and duodenum uptake of barium sulfate occurs almost immediately after oral administration. Small intestine uptake is dependent on gastric emptying rate and viscosity of the preparation; it may be delayed 15-90 minutes post ingestion. Small intestine (enteroclysis studies) uptake is immediate, following direct instillation. Colon and distal small intestine uptake are dependent on patient positioning. Hydrostatic pressure also determines the rate and degree of opacification.
Route of Elimination
Barium sulfate is excreted unchanged in the feces.
Clearance
The rate of excretion of barium sulfate is dependent on the route of administration and the status of peristaltic activity and motility of the gastrointestinal tract.
/In female beagle dog,/ (131)barium sulfate was found to be cleared from lung, with a biological half-time of 8-9 days ... . This indicated some solubility ... In body fluids, possibly in colloidal form. ... Barium sulfate ... Clearance rate /depends/ on specific surface area of inhaled particles and was lower for heat treated than for untreated particles.
Friberg, L., Nordberg, G.F., Kessler, E. and Vouk, V.B. (eds). Handbook of the Toxicology of Metals. 2nd ed. Vols I, II.: Amsterdam: Elsevier Science Publishers B.V., 1986., p. V2 87
In an extensive study, temperature and pulse rate measurements were taken as an indication of an acute inflammatory response for 291 humans administered a single unstated dose of a 50% w/v barium sulfate suspension for bronchographic purposes. The method of administration was unstated, but the suspension was presumed to have been instilled into the trachea and then blown into the lungs. In 154 patients, there was radiological evidence of the presence of barium sulfate in the bronchial tree at the time of the last available X-ray (various time points ranging from < 1 week to > 1 year after administration); in 135 patients, on the other hand, there was no radiological evidence of residual barium sulfate in the lungs 1 year after bronchography. Forty-one of these patients exhibited complete elimination of the barium sulfate from the lungs within 1 week; it was stated that in some of these patients, this clearance occurred within 24 hr.
International Programme on Chemical Safety; Concise International Chemical Assessment Document Number 33: Barium and Barium Compounds (2001). Available from, as of August 5, 2010: https://www.inchem.org/pages/cicads.html
After inhalation exposure of rats to a BaSO4 aerosol at 40 mg/cu m, 5 hr daily for 2 months, lymphatic transport was slight. The skeletal concentration of Ba was 0.8-1.5 mg/g dry substance (10-100 times the pulmonary concentration). Skeletal uptake decreased somewhat with advancing age.
Norderg, G.F. et al; Handbook on the Toxicology of Metals 3rd ed. Academic Press, Burlington, MA. 2007, p. 410
Of BaSO4 injected intratracheally, about 5% of the initial dose was recovered in the urine during the first 10 days after administration.
Norderg, G.F. et al; Handbook on the Toxicology of Metals 3rd ed. Academic Press, Burlington, MA. 2007, p. 410
For more Absorption, Distribution and Excretion (Complete) data for Barium sulfate (11 total), please visit the HSDB record page.
Barium sulfate is poorly water soluble and shows negligible levels of absorption from the gastrointestinal tract following both oral or rectal administration. In healthy subjects, orally administered barium sulfate is generally excreted within 24 hours. Rectally administered barium sulfate is eliminated with clearance of the enema.
The in vivo solubility in rats of barium chloride, barium carbonate, barium sulfate, and barium fused in clay /was studied/. The chloride and the carbonate disappeared rapidly from the injection site. The sulfate dissolved more slowly; a half-life of 26 days was calculated. The fused clay was largely retained, the half-life being calculated as 1390 days.
American Conference of Governmental Industrial Hygienists. Documentation of the TLV's and BEI's with Other World Wide Occupational Exposure Values. CD-ROM Cincinnati, OH 45240-4148 2010.
... In rats barium sulfate disappeared from the injection site with a half-life of 26 days ...
WHO; Environ Health Criteria 107: Barium (1990). Available from, as of August 3, 2010: https://www.inchem.org/pages/ehc.html
In Syrian hamsters, barium sulfate was found to be cleared from the lungs with a biological half-life of 8-9 days ...
WHO; Environ Health Criteria 107: Barium (1990). Available from, as of August 3, 2010: https://www.inchem.org/pages/ehc.html
(131)BaSO4 was found to be cleared from the lungs, with a biological half-life time of 8-9 days, through absorption into the general circulation with subsequent urinary clearance.
Norderg, G.F. et al; Handbook on the Toxicology of Metals 3rd ed. Academic Press, Burlington, MA. 2007, p. 409
Measurements of the clearance of tracer levels of (131)barium sulfate (estimated maximum initial burden of 90 ug or 0.09 mg/kg from a 30 to 90 minute exposure) from the respiratory tract of female beagle dogs (10 + or - 1 kg) were performed. The biological half-life was eight days.
USEPA; Drinking Water Criteria Document for Barium (Draft) p.III-8 (1985) TR-540-60F
/Barium/ sulfate ... /has a calculated/ half-life of 26 days /in rats/.
American Conference of Governmental Industrial Hygienists. Documentation of the TLV's and BEI's with Other World Wide Occupational Exposure Values. CD-ROM Cincinnati, OH 45240-4148 2010.
Barium sulfate is a heavy metal with a high atomic number (Z=56) and a K shell binding energy (K-edge of 37.4 keV) very close to that of most diagnostic x-ray beams. Due to these characteristics, barium is an ideal medium for the absorption of x-rays. Barium sulfate is essentially not absorbed from the GI tract nor metabolized in the body. Barium sulfate is used to fill the gastrointestinal tract lumen or to coat the mucosal surface and is administered orally, rectally, or instilled into an enterostomy tube or catheter,. Barium sulfate enhances delineation of the GI tract. The barium suspension covers the mucosal surface of the GI tract, allowing its shape, distensibility, motion, integrity, continuity, location within the torso, relationship to other organs to be closely examined. Various abnormalities, such as benign or malignant tumors, ulcers, strictures, diverticula, inflammation or infection, altered motility, displacement and other pathology can thereby be identified,. At lower concentrations (higher dilution), barium enhances the conspicuity of the GI tract to differentiate the GI tract from various abdominal organs in computed tomography examinations (CT scans) of the abdomen. Improved delineation of the gastrointestinal tract lumen and mucosa may be reached by contrast provided by gas (by the addition of bicarbonate or gas-filled balloons) in addition to the barium. This is known as a _double-contrast procedure_. Osmotically active agents (for example, sorbitol) are also used to induce fluid accumulation and distension of the GI system to enhance visualization.
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

ABOUT THIS PAGE
68
PharmaCompass offers a list of Barium Sulfate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Barium Sulfate manufacturer or Barium Sulfate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Barium Sulfate manufacturer or Barium Sulfate supplier.
PharmaCompass also assists you with knowing the Barium Sulfate API Price utilized in the formulation of products. Barium Sulfate API Price is not always fixed or binding as the Barium Sulfate Price is obtained through a variety of data sources. The Barium Sulfate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Barium Sulfate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Barium Sulfate, including repackagers and relabelers. The FDA regulates Barium Sulfate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Barium Sulfate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Barium Sulfate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Barium Sulfate supplier is an individual or a company that provides Barium Sulfate active pharmaceutical ingredient (API) or Barium Sulfate finished formulations upon request. The Barium Sulfate suppliers may include Barium Sulfate API manufacturers, exporters, distributors and traders.
click here to find a list of Barium Sulfate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Barium Sulfate Drug Master File in Japan (Barium Sulfate JDMF) empowers Barium Sulfate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Barium Sulfate JDMF during the approval evaluation for pharmaceutical products. At the time of Barium Sulfate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Barium Sulfate suppliers with JDMF on PharmaCompass.
A Barium Sulfate CEP of the European Pharmacopoeia monograph is often referred to as a Barium Sulfate Certificate of Suitability (COS). The purpose of a Barium Sulfate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Barium Sulfate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Barium Sulfate to their clients by showing that a Barium Sulfate CEP has been issued for it. The manufacturer submits a Barium Sulfate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Barium Sulfate CEP holder for the record. Additionally, the data presented in the Barium Sulfate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Barium Sulfate DMF.
A Barium Sulfate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Barium Sulfate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Barium Sulfate suppliers with CEP (COS) on PharmaCompass.
A Barium Sulfate written confirmation (Barium Sulfate WC) is an official document issued by a regulatory agency to a Barium Sulfate manufacturer, verifying that the manufacturing facility of a Barium Sulfate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Barium Sulfate APIs or Barium Sulfate finished pharmaceutical products to another nation, regulatory agencies frequently require a Barium Sulfate WC (written confirmation) as part of the regulatory process.
click here to find a list of Barium Sulfate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Barium Sulfate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Barium Sulfate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Barium Sulfate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Barium Sulfate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Barium Sulfate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Barium Sulfate suppliers with NDC on PharmaCompass.
Barium Sulfate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Barium Sulfate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Barium Sulfate GMP manufacturer or Barium Sulfate GMP API supplier for your needs.
A Barium Sulfate CoA (Certificate of Analysis) is a formal document that attests to Barium Sulfate's compliance with Barium Sulfate specifications and serves as a tool for batch-level quality control.
Barium Sulfate CoA mostly includes findings from lab analyses of a specific batch. For each Barium Sulfate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Barium Sulfate may be tested according to a variety of international standards, such as European Pharmacopoeia (Barium Sulfate EP), Barium Sulfate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Barium Sulfate USP).
