


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
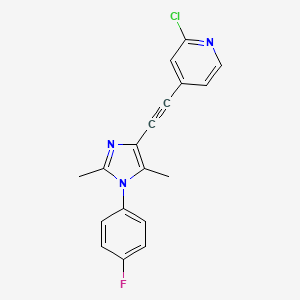

1. 2-chloro-4-(1-(4-fluorophenyl)-2,5-dimethyl-1h-imidazol-4-ylethynyl)pyridine
2. Ro4917523
1. 802906-73-6
2. Ctep Derivative
3. Rg7090
4. 2-chloro-4-((1-(4-fluorophenyl)-2,5-dimethyl-1h-imidazol-4-yl)ethynyl)pyridine
5. Rg-7090
6. Ro4917523
7. Ro-4917523
8. 2-chloro-4-[2-[1-(4-fluorophenyl)-2,5-dimethylimidazol-4-yl]ethynyl]pyridine
9. Noe-101
10. 3110e3ao8s
11. 1034442-21-1 (free Base)
12. 2-chloro-4-(2-(1-(4-fluorophenyl)-2,5-dimethyl-1h-imidazol-4-yl)ethynyl)pyridine
13. Pyridine, 2-chloro-4-(2-(1-(4-fluorophenyl)-2,5-dimethyl-1h-imidazol-4-yl)ethynyl)-
14. 1034442-21-1
15. Basimglurant [usan:inn]
16. Unii-3110e3ao8s
17. 2-chloro-4-[2-[1-(4-fluorophenyl)-2,5-dimethyl-1h-imidazol-4-yl]ethynyl]pyridine
18. Basimglurant [inn]
19. Rg7090basimglurant
20. Rg 7090
21. Ro 4917523
22. Basimglurant (usan/inn)
23. Basimglurant [usan]
24. Basimglurant [who-dd]
25. Schembl560590
26. Gtpl9309
27. Chembl3301626
28. Dtxsid801025734
29. Hms3741c15
30. Bcp15864
31. Zinc6716839
32. Bdbm50071374
33. Mfcd25976719
34. Rg7090rg7090
35. Compound 2 [pmid: 25565255]
36. Cs-3388
37. Db11833
38. Ncgc00390716-02
39. Ac-35776
40. As-51978
41. Hy-15446
42. Ds-015787
43. D10864
44. P14045
45. A916083
46. Q18209947
47. 1-(4-fluorophenyl)-4-[(2-chloro-4-pyridyl)ethynyl]-2,5-dimethyl-1h-imidazole
48. 2-chloro-4-[1-(4-fluoro-phenyl)-2,5-dimethyl-1h-imidazol-4-ylethynyl]-pyridine
| Molecular Weight | 325.8 g/mol |
|---|---|
| Molecular Formula | C18H13ClFN3 |
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 325.0782033 g/mol |
| Monoisotopic Mass | 325.0782033 g/mol |
| Topological Polar Surface Area | 30.7 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 465 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


