
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
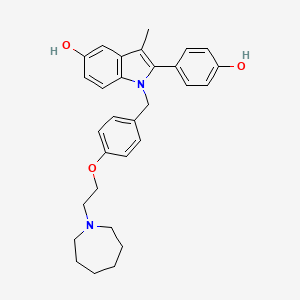

1. Bazedoxifene Acetate
2. Tse 424
3. Tse-424
4. Tse424
5. Way-140424
1. 198481-32-2
2. Bazedoxifene [inn]
3. Tse-424
4. Bazedoxifene Free Base
5. 1-(4-(2-(azepan-1-yl)ethoxy)benzyl)-2-(4-hydroxyphenyl)-3-methyl-1h-indol-5-ol
6. 1h-indol-5-ol, 1-[[4-[2-(hexahydro-1h-azepin-1-yl)ethoxy]phenyl]methyl]-2-(4-hydroxyphenyl)-3-methyl-
7. Q16tt9c5bk
8. Chembl46740
9. 198481-32-2 (free Base)
10. 1-[[4-[2-(azepan-1-yl)ethoxy]phenyl]methyl]-2-(4-hydroxyphenyl)-3-methylindol-5-ol
11. Way 140424
12. Bazedoxifeno
13. 1-[4-(2-azepan-1-yl-ethoxy)-benzyl]-2-(4-hydroxy-phenyl)-3-methyl-1h-indol-5-ol
14. 1h-indol-5-ol, 1-((4-(2-(hexahydro-1h-azepin-1-yl)ethoxy)phenyl)methyl)-2-(4-hydroxyphenyl)-3-methyl-
15. Unii-q16tt9c5bk
16. Bazedoxifeno [inn-spanish]
17. 1-{4-[2-(azepan-1-yl)ethoxy]benzyl}-2-(4-hydroxyphenyl)-3-methyl-1h-indol-5-ol
18. Bazedoxifene [mi]
19. Bazedoxifene [vandf]
20. Schembl41935
21. Bazedoxifene [who-dd]
22. Gtpl7355
23. Bazedoxifene [ema Epar]
24. Dtxsid70173593
25. Chebi:135947
26. Ex-a5409
27. Hy-a0031
28. Zinc1895505
29. Bdbm50099585
30. Akos030255808
31. Ak R215 Component Bazedoxifene
32. Ak-r215 Component Bazedoxifene
33. Cs-0932
34. Db06401
35. Sb19326
36. 1-[[4-[2-(azepan-1-yl)ethoxy]phenyl]methyl]-2-(4-hydroxyphenyl)-3-methyl-indol-5-ol
37. 1-((4-(2-hexahydro-1h-azepin-1-yl)ethoxy)phenyl)methyl)-2-(4-hydroxyphenyl)-3-methyl-1h-indol-5-ol
38. As-78494
39. Us8815934, No. 98
40. A15019
41. D94589
42. Ab01566901_01
43. A879977
44. J-012822
45. Q4875166
46. 1-(p-(2-(hexahydro-1h-azepin-1-yl)ethoxy)benzyl)-2-(p-hydroxyphenyl)-3-methylindol-5-ol
| Molecular Weight | 470.6 g/mol |
|---|---|
| Molecular Formula | C30H34N2O3 |
| XLogP3 | 6.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 470.25694295 g/mol |
| Monoisotopic Mass | 470.25694295 g/mol |
| Topological Polar Surface Area | 57.9 Ų |
| Heavy Atom Count | 35 |
| Formal Charge | 0 |
| Complexity | 623 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for following conditions alone or in combination with conjugated estrogens in women with a uterus: - Treatment of moderate to severe vasomotor symptoms associated with menopause - Prevention of postmenopausal osteoporosis
FDA Label
Conbriza is indicated for the treatment of postmenopausal osteoporosis in women at increased risk of fracture. A significant reduction in the incidence of vertebral fractures has been demonstrated; efficacy on hip fractures has not been established.
When determining the choice of Conbriza or other therapies, including oestrogens, for an individual postmenopausal woman, consideration should be given to menopausal symptoms, effects on uterine and breast tissues, and cardiovascular risks and benefits.
Selective Estrogen Receptor Modulators
A structurally diverse group of compounds distinguished from ESTROGENS by their ability to bind and activate ESTROGEN RECEPTORS but act as either an agonist or antagonist depending on the tissue type and hormonal milieu. They are classified as either first generation because they demonstrate estrogen agonist properties in the ENDOMETRIUM or second generation based on their patterns of tissue specificity. (Horm Res 1997;48:155-63) (See all compounds classified as Selective Estrogen Receptor Modulators.)
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
G03XC02
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03X - Other sex hormones and modulators of the genital system
G03XC - Selective estrogen receptor modulators
G03XC02 - Bazedoxifene
Absorption
Bazedoxifene is rapidly absorbed with a tmax of approximately 2 hours and exhibits a linear increase in plasma concentrations for single doses from 0.5 mg up to 120 mg and multiple daily doses from 1 mg to 80 mg. The absolute bioavailability of bazedoxifene is approximately 6%.
Route of Elimination
The major route of elimination of radio-labelled bazedoxifene is the faeces, and less than 1% of the dose is eliminated in urine.
Volume of Distribution
Following intravenous administration of a 3 mg dose of bazedoxifene, the volume of distribution is 14.7 3.9 l/kg.
Clearance
The apparent oral clearance of bazedoxifene is approximately 4 to 5 l/h/kg.
Glucuronidation is the major metabolic pathway. After peroral application, bazedoxifene is metabolized by UDP-glucuronosyltransferases (UGTs) to bazedoxifene-4'-glucuronide (M4) and bazedoxifene-5-glucuronide (M5).Little or no cytochrome P450-mediated metabolism is evident. The concentrations of this glucuronide are approximately 10-fold higher than those of unchanged active substance in plasma.
~30 hours.
Bazedoxifene belongs to a class of compounds known as selective estrogen receptor modulators (SERMs). Bazedoxifene acts as both an oestrogen-receptor agonist and/or antagonist, depending upon the cell and tissue type and target genes. Bazedoxifene decreases bone resorption and reduces biochemical markers of bone turnover to the premenopausal range. These effects on bone remodelling lead to an increase in bone mineral density (BMD), which in turn contributes to a reduction in the risk of fractures. Bazedoxifene functions primarily as an oestrogen-receptor antagonist in uterine and breast tissues.
Global Sales Information

Market Place

ABOUT THIS PAGE
64
PharmaCompass offers a list of Bazedoxifene API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Bazedoxifene manufacturer or Bazedoxifene supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Bazedoxifene manufacturer or Bazedoxifene supplier.
PharmaCompass also assists you with knowing the Bazedoxifene API Price utilized in the formulation of products. Bazedoxifene API Price is not always fixed or binding as the Bazedoxifene Price is obtained through a variety of data sources. The Bazedoxifene Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Bazedoxifene manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Bazedoxifene, including repackagers and relabelers. The FDA regulates Bazedoxifene manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Bazedoxifene API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Bazedoxifene supplier is an individual or a company that provides Bazedoxifene active pharmaceutical ingredient (API) or Bazedoxifene finished formulations upon request. The Bazedoxifene suppliers may include Bazedoxifene API manufacturers, exporters, distributors and traders.
click here to find a list of Bazedoxifene suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Bazedoxifene DMF (Drug Master File) is a document detailing the whole manufacturing process of Bazedoxifene active pharmaceutical ingredient (API) in detail. Different forms of Bazedoxifene DMFs exist exist since differing nations have different regulations, such as Bazedoxifene USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Bazedoxifene DMF submitted to regulatory agencies in the US is known as a USDMF. Bazedoxifene USDMF includes data on Bazedoxifene's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Bazedoxifene USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Bazedoxifene suppliers with USDMF on PharmaCompass.
Bazedoxifene Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Bazedoxifene GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Bazedoxifene GMP manufacturer or Bazedoxifene GMP API supplier for your needs.
A Bazedoxifene CoA (Certificate of Analysis) is a formal document that attests to Bazedoxifene's compliance with Bazedoxifene specifications and serves as a tool for batch-level quality control.
Bazedoxifene CoA mostly includes findings from lab analyses of a specific batch. For each Bazedoxifene CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Bazedoxifene may be tested according to a variety of international standards, such as European Pharmacopoeia (Bazedoxifene EP), Bazedoxifene JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Bazedoxifene USP).
