
Synopsis
Synopsis
0
VMF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Aerobec
2. Aerobec Forte
3. Aldecin
4. Apo-beclomethasone
5. Ascocortonyl
6. Asmabec Clickhaler
7. Beclamet
8. Beclazone
9. Beclazone Easy Breathe
10. Beclo Asma
11. Beclo Azu
12. Beclocort
13. Becloforte
14. Beclomet
15. Beclometasone
16. Beclomethasone
17. Beclorhinol
18. Becloturmant
19. Beclovent
20. Becodisk
21. Becodisks
22. Beconase
23. Beconase Aq
24. Becotide
25. Bemedrex Easyhaler
26. Bronchocort
27. Dipropionate, Beclomethasone
28. Ecobec
29. Filair
30. Filair Forte
31. Junik
32. Nasobec Aqueous
33. Prolair
34. Propaderm
35. Qvar
36. Respocort
37. Sanasthmax
38. Sanasthmyl
39. Vancenase
40. Vanceril
41. Ventolair
42. Viarin
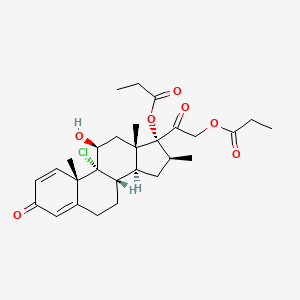
1. Beclometasone Dipropionate
2. 5534-09-8
3. Beclovent
4. Beconase
5. Vancenase
6. Sanasthmax
7. Beclazone
8. Beclomet
9. Vanceril
10. Becloforte
11. Beclorhinol
12. Entyderma
13. Korbutone
14. Propaderm
15. Sanasthmyl
16. Aerobec
17. Aldecin
18. Qvar
19. Beclacin
20. Ventolair
21. Viarox
22. Propaderm Forte
23. Rino-clenil
24. Inalone R
25. Clenil-a
26. Beclometasone 17,21-dipropionate
27. Qnasl
28. Becotide
29. Sch 18020w
30. Beconase Aq
31. Beclate
32. Becodisks
33. Benconase
34. Chebi:3002
35. (11beta,16beta)-9-chloro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)pregna-1,4-diene-3,20-dione
36. Inalone O
37. Sch 8020w
38. Sgx-201
39. Sgx-202
40. Sgx-203
41. Sch-18020w
42. 9-chloro-11beta,17,21-trihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate
43. 9-chloro-11beta-hydroxy-16beta-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate
44. 5b307s63b2
45. Nsc-755901
46. Becloturmant
47. Respocort
48. Belchlorhinol
49. Sanasthymyl
50. Anceron
51. Becloval
52. [2-[(8s,9r,10s,11s,13s,14s,16s,17r)-9-chloro-11-hydroxy-10,13,16-trimethyl-3-oxo-17-propanoyloxy-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] Propanoate
53. Pregna-1,4-diene-3,20-dione, 9-chloro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)-, (11.beta.,16.beta.)-
54. Viarex
55. Vancenase Aq
56. Alanase
57. Aldecina
58. Aldecine
59. Atomase
60. Rhinosol
61. Turbinal
62. Clenil
63. Spir
64. Beclocort Nasel
65. Beclomet Nasal
66. Rhino Clenil
67. (8s,9r,10s,11s,13s,14s,16s,17r)-9-chloro-11-hydroxy-10,13,16-trimethyl-3-oxo-17-[2-(propionyloxy)acetyl]-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3h-cyclopenta[a]phenanthren-17-yl Propionate
68. 9-chloro-11.beta.,17,21-trihydroxy-16.beta.-methylpregna-1,4-diene-3,20-dione 17,21-dipropionate
69. 9-chloro-16beta-methyl-11beta,17,21-trihydroxypregna-1,4-diene-3,20-dione 17,21-dipropionate
70. Qvar 80
71. Vanceril Double Strength
72. Beconasol
73. Inalone
74. Menaderm
75. Qvar 40
76. Orbec
77. Beclovent (tn)
78. Ncgc00094596-03
79. Beclomethasone Dipropionate [usan]
80. Vanceril (tn)
81. Beclazone 250
82. Prestwick_990
83. Einecs 226-886-0
84. Kw-053
85. Mfcd00135613
86. Nasobec
87. Orbeshield
88. Topster
89. Qnasl (tn)
90. Clenil A
91. Beclometasone Dipropionato
92. Prestwick0_000855
93. Prestwick1_000855
94. Prestwick2_000855
95. Prestwick3_000855
96. Beclomethasone-dipropionate
97. Schembl6890
98. Dsstox_cid_28656
99. Dsstox_rid_82926
100. Propionic Acid Beclomethasone
101. Dsstox_gsid_48730
102. Bspbio_000869
103. Mls002154034
104. Spbio_002790
105. Bpbio1_000957
106. Gtpl5894
107. Chembl1200500
108. Dtxsid3048730
109. Unii-5b307s63b2
110. Beclomethasone Dipropionate (usp)
111. Hms1570l11
112. Hms2097l11
113. Hms2235o23
114. Hms3259h03
115. Hms3714l11
116. Beclometasone Dipropionate (jp17)
117. Beclomethasone Dipropionate Anhydrous
118. Zinc3938744
119. Tox21_113168
120. Hy-13571a
121. S3078
122. Akos015951275
123. Ac-2159
124. Ccg-220855
125. Cs-1503
126. Db00394
127. Nc00610
128. Nsc 755901
129. Beclometasone Dipropionate [jan]
130. Beclomethasone Dipropionate [mi]
131. Beclomethasone Dipropionate [usan:usp]
132. Ncgc00179392-01
133. (11beta,16beta)-9-chloro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-diene-17,21-diyl Dipropanoate
134. Cpd001233361
135. Pregna-1,4-diene-3,20-dione, 9-chloro-11-hydroxy-16-methyl-17,21-bis(1-oxopropoxy)-, (11beta,16beta)-
136. Pregna-1,4-diene-3,20-dione, 9-chloro-16-beta-methyl-11-beta,17,21-trihydroxy-, 17,21-dipropionate
137. Smr001233361
138. Beclometasone Dipropionate [who-dd]
139. Beclometasone Dipropionate [who-ip]
140. Beclometasone Dipropionate Anhydrous
141. Beclomethasone 17,21-dipropionate
142. Beclomethasone Dipropionate [vandf]
143. Cas-5534-09-8
144. Beclometasone Dipropionate, Anhydrous
145. Beclomethasone 17,21-diproprionate
146. Beclomethasone Dipropionate [usp-rs]
147. Betamethasone Dipropionate Impurity E
148. B4464
149. C07813
150. D00689
151. D89082
152. Beclometasone Dipropionate [ep Monograph]
153. Beclometasoni Dipropionas [who-ip Latin]
154. Beclomethasone Dipropionate [orange Book]
155. 534b098
156. Beclomethasone Dipropionate [usp Impurity]
157. Beclomethasone Dipropionate [usp Monograph]
158. Q421475
159. Trimbow Component Beclomethasone Dipropionate
160. Brd-k97810537-001-03-7
161. Brd-k97810537-001-13-6
162. Beclometasone Dipropionate, Anhydrous [ep Impurity]
163. Beclomethasone Dipropionate, Analytical Standard, For Drug Analysis
164. Beclometasone (beclomethasone) Dipropionate, British Pharmacopoeia (bp) Assay Standard
165. Beclomethasone Dipropionate, Anhydrous, European Pharmacopoeia (ep) Reference Standard
166. Beclomethasone Dipropionate, Pharmaceutical Secondary Standard; Certified Reference Material
167. Beclomethasone Dipropionate, United States Pharmacopeia (usp) Reference Standard
168. [(8s,9r,10s,11s,13s,14s,16s,17r)-9-chloro-11-hydroxy-10,13,16-trimethyl-3-oxo-17-(2-propanoyloxyacetyl)-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl] Propanoate
169. 2-[(1r,2s,10s,11s,13s,14r,15s,17s)-1-chloro-17-hydroxy-2,13,15-trimethyl-5-oxo-14-(propanoyloxy)tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-14-yl]-2-oxoethyl Propanoate
170. 9alpha-chloro-16beta-methyl-3,20-dioxo-1,4-pregnadiene-11beta,17,21-triol 17,21-dipropionate
171. Beclometasone Dipropionate For Peak Identification, European Pharmacopoeia (ep) Reference Standard
172. Beclometasone Dipropionate For System Suitability, European Pharmacopoeia (ep) Reference Standard
| Molecular Weight | 521.0 g/mol |
|---|---|
| Molecular Formula | C28H37ClO7 |
| XLogP3 | 3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 8 |
| Exact Mass | 520.2227812 g/mol |
| Monoisotopic Mass | 520.2227812 g/mol |
| Topological Polar Surface Area | 107 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 1050 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Qnasl |
| PubMed Health | Beclomethasone |
| Drug Classes | Anti-Inflammatory |
| Drug Label | Beclomethasone dipropionate USP, the active component of QNASL Nasal Aerosol, is an anti-inflammatory steroid having the chemical name 9-chloro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione 17, 21-dipropionate and the following chemica... |
| Active Ingredient | Beclomethasone dipropionate |
| Dosage Form | Aerosol, metered |
| Route | Nasal |
| Strength | 0.08mg/actuation |
| Market Status | Prescription |
| Company | Teva Branded Pharm |
| 2 of 4 | |
|---|---|
| Drug Name | Qvar 40 |
| Active Ingredient | Beclomethasone dipropionate |
| Dosage Form | Aerosol, metered |
| Route | Inhalation |
| Strength | 0.04mg/inh |
| Market Status | Prescription |
| Company | Teva Branded Pharm |
| 3 of 4 | |
|---|---|
| Drug Name | Qnasl |
| PubMed Health | Beclomethasone |
| Drug Classes | Anti-Inflammatory |
| Drug Label | Beclomethasone dipropionate USP, the active component of QNASL Nasal Aerosol, is an anti-inflammatory steroid having the chemical name 9-chloro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione 17, 21-dipropionate and the following chemica... |
| Active Ingredient | Beclomethasone dipropionate |
| Dosage Form | Aerosol, metered |
| Route | Nasal |
| Strength | 0.08mg/actuation |
| Market Status | Prescription |
| Company | Teva Branded Pharm |
| 4 of 4 | |
|---|---|
| Drug Name | Qvar 40 |
| Active Ingredient | Beclomethasone dipropionate |
| Dosage Form | Aerosol, metered |
| Route | Inhalation |
| Strength | 0.04mg/inh |
| Market Status | Prescription |
| Company | Teva Branded Pharm |
Indicated for oral inhalation use in the maintenance treatment of asthma as prophylactic therapy in patients 5 years of age and older. The aerosol form of beclomethasone diproprionate is not indicated for the relief of acute bronchospasm. Indicated for intranasal use to relieve the symptoms of seasonal or perennial allergic and nonallergic (vasomotor) rhinitis and prevent the recurrence of nasal polyps following surgical removal. Indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in patients 13 years of age and older. Corticosteroid-responsive dermatoses include psoriasis, contact dermatitis (dermatitis venenata), atopic dermatitis (infantile eczema, allergic dermatitis), neurodermatitis (lichen simplex chronicus, lichen planus, eczema, eczematous dermatitis), intertrigo, dyshidroses (pompholyx), seborrheic dermatitis, exfoliative dermatitis, solar dermatitis, stasis dermatitis, and anogenital and senile pruritus.
Inflammatory conditions, including asthma, dermatoses, and allergic rhinitis, involve the activation of cascades by inflammatory mediators. Inflammation is a primary defense mechanism and the homeostatic response of the immune system; however, a prolonged inflammatory response in certain disorders may lead to tissue damage, pain, and swelling. Beclomethasone dipropionate works by attenuating the inflammatory responses associated with asthma, allergic rhinitis, nasal polyps, and corticosteroid-responsive dermatoses. It suppresses the actions of inflammatory cells, such as mast cells, eosinophils, basophils, lymphocytes, macrophages, and neutrophils. It also inhibits the release of inflammatory mediators, such as histamine, eicosanoids, leukotrienes, and cytokines. Beclomethasone dipropionate is reported to exhibit potent topical activity while possessing low systemic effects. Beclomethasone dipropionate is a corticosteroid drug with anti-inflammatory and vasoconstrictive effects used to treat chronic inflammatory processes such as asthma, allergic rhinitis, corticosteroid-responsive dermatoses. When inhaled, it improves lung function, decreases airway hyper-reactivity, and reduces the severity of asthmatic symptoms. Although inhaled corticosteroids, including beclomethasone dipropionate, are reported to mainly act locally in the lungs, systemic effects such as disruption of hypothalamic-pituitary-adrenal (HPA) axis function, bone turnover, osteoporosis, and growth suppression may still be observed with chronic use or high dose administration. There were varying findings from clinical studies examining the effect of beclomethasone dipropionate on growth suppression in pediatric patients. It was shown to suppress the hypothalamo-pituitary-adrenal (HPA) axis in a dose-dependent manner. HPA axis is a central hormonal response system to stress and activation of HPA axis leads to the production of endogenous steroid hormone production. Long-term use of high-dose systemic corticosteroids, including those inhaled, was often associated with signs and symptoms of adrenal insufficiency when exposed to stress conditions, such as trauma, surgery, or infections. As corticosteroids work by suppressing the immune system, there may be an increased risk for developing infections. Cases of _Candida albicans_ infection of the mouth and throat have been reported with inhaled beclomethasone dipropionate therapy.
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Glucocorticoids
A group of CORTICOSTEROIDS that affect carbohydrate metabolism (GLUCONEOGENESIS, liver glycogen deposition, elevation of BLOOD SUGAR), inhibit ADRENOCORTICOTROPIC HORMONE secretion, and possess pronounced anti-inflammatory activity. They also play a role in fat and protein metabolism, maintenance of arterial blood pressure, alteration of the connective tissue response to injury, reduction in the number of circulating lymphocytes, and functioning of the central nervous system. (See all compounds classified as Glucocorticoids.)
Anti-Asthmatic Agents
Drugs that are used to treat asthma. (See all compounds classified as Anti-Asthmatic Agents.)
Absorption
Following oral inhalation of 320 mcg of beclomethasone dipropionate (BDP), the Cmax was 88 pg/mL and it was reached after 0.5 at post-administration. The mean Cmax of the major and most active metabolite, beclomethasone-17-monopropionate (17-BMP), was 1419 pg/mL at 0.7 hour post-dosing. In another pharmacokinetic study, the AUC of BDP and 17-BMP were 6660 and 6185 pgxh/mL, respectively. The Cmax was 35356 pg/mL for BDP and 2633 pg/mL for 17-BMP, and and the median time to reach these concentrations (Tmax) was 0.2 hours. In the same study, the AUC of 17-BMP following oral and intranasal administration were 10158 and 3660 pgxh/mL, respectively. The Cmax of 17-BMP following oral and intranasal administration were 703 and 310 pg/mL, respectively, and the Tmax was 4 hours. The total bioavailability of 17-BMP following oral and intranasal administration were 41% and 44%, respectively.
Route of Elimination
Regardless of the route of administration, beclomethasone dipropionate and its metabolites are predominantly excreted in the feces, with less than 10% of the drug and its metabolites being excreted in the urine.
Volume of Distribution
Following intravenous administration, the steady-state volume of distribution was 20 L for beclomethasone dipropionate and 424 L for the active metabolite, beclomethasone-17-monopropionate.
Clearance
Following intravenous administration, the clearance of beclomethasone dipropionate and 17-BMP were 150 L/h and 120 L/h, respectively.
During absorption, beclomethasone dipropionate is undergoes rapid and extensive hydrolysis mediated by esterases CYP3A to form beclomethasone-17-monopropionate (17-BMP), beclomethasone-21-monopropionate (21-BMP), and beclomethasone (BOH). 17-BMP is the major active metabolite with the most potent anti-inflammatory activity. About 95% of the total beclomethasone dipropionate administered via oral inhalation undergoes presystemic conversion to form 17-BMP in the lung.
Following intravenous administration, the half life of beclomethasone dipropionate was 0.5 hours while the half life of the active metabolite 17-BMP was 2.7 hours. Following oral and intranasal administration, the half life of 17-BMP was 8.8 and 5.7 hours, respectively.
Beclomethasone dipropionate is a corticosteroid and prodrug that is rapidly activated by hydrolysis to the active monoester, 17 monopropionate (17-BMP), which mediates anti-inflammatory actions. 17-BMP has been shown _in vitro_ to exhibit a binding affinity for the human glucocorticoid receptor which is approximately 13 times that of dexamethasone and 25 times that of beclomethasone dipropionate. Upon binding of the ligand, the glucocorticoid receptors dimerize and translocate into the nucleus, where they subsequently bind to glucocorticoid response elements (GRE) on glucocorticoid-responsive genes, leading to changes in transcription. There are several proposed mechanisms for the anti-inflammatory action of corticosteroids. Corticosteroids may work by increasing the transcription of genes coding for anti-inflammatory proteins, including lipocortin-1 and interleukin-10. Corticosteroids were also shown to inhibit the expression of multiple genes that encode pro-inflammatory factors, such as cytokines, chemokines, and adhesion molecules, that are activated during the chronic inflammatory process. This is thought to be due to the direct inhibitory interaction between activated glucocorticoid receptors and activated pro-inflammatory transcription factors, such as nuclear factor-kappa B and activator protein-1. Chronic inflammation is often characterized by enhanced expression of these transcription factors that bind to and activate coactivator molecules, which then acetylate core histones to switch on gene transcription to further amplify the inflammatory process. Corticosteroids suppress the multiple inflammatory gene expression by promoting histone deacetylation, resulting in tighter coiling of DNA and reduced access of transcription factors to their binding sites.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
73
PharmaCompass offers a list of Beclomethasone Dipropionate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Beclomethasone Dipropionate manufacturer or Beclomethasone Dipropionate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Beclomethasone Dipropionate manufacturer or Beclomethasone Dipropionate supplier.
PharmaCompass also assists you with knowing the Beclomethasone Dipropionate API Price utilized in the formulation of products. Beclomethasone Dipropionate API Price is not always fixed or binding as the Beclomethasone Dipropionate Price is obtained through a variety of data sources. The Beclomethasone Dipropionate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Beclomethasone Dipropionate Monohydrate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Beclomethasone Dipropionate Monohydrate, including repackagers and relabelers. The FDA regulates Beclomethasone Dipropionate Monohydrate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Beclomethasone Dipropionate Monohydrate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Beclomethasone Dipropionate Monohydrate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Beclomethasone Dipropionate Monohydrate supplier is an individual or a company that provides Beclomethasone Dipropionate Monohydrate active pharmaceutical ingredient (API) or Beclomethasone Dipropionate Monohydrate finished formulations upon request. The Beclomethasone Dipropionate Monohydrate suppliers may include Beclomethasone Dipropionate Monohydrate API manufacturers, exporters, distributors and traders.
click here to find a list of Beclomethasone Dipropionate Monohydrate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Beclomethasone Dipropionate Monohydrate DMF (Drug Master File) is a document detailing the whole manufacturing process of Beclomethasone Dipropionate Monohydrate active pharmaceutical ingredient (API) in detail. Different forms of Beclomethasone Dipropionate Monohydrate DMFs exist exist since differing nations have different regulations, such as Beclomethasone Dipropionate Monohydrate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Beclomethasone Dipropionate Monohydrate DMF submitted to regulatory agencies in the US is known as a USDMF. Beclomethasone Dipropionate Monohydrate USDMF includes data on Beclomethasone Dipropionate Monohydrate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Beclomethasone Dipropionate Monohydrate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Beclomethasone Dipropionate Monohydrate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Beclomethasone Dipropionate Monohydrate Drug Master File in Japan (Beclomethasone Dipropionate Monohydrate JDMF) empowers Beclomethasone Dipropionate Monohydrate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Beclomethasone Dipropionate Monohydrate JDMF during the approval evaluation for pharmaceutical products. At the time of Beclomethasone Dipropionate Monohydrate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Beclomethasone Dipropionate Monohydrate suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Beclomethasone Dipropionate Monohydrate Drug Master File in Korea (Beclomethasone Dipropionate Monohydrate KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Beclomethasone Dipropionate Monohydrate. The MFDS reviews the Beclomethasone Dipropionate Monohydrate KDMF as part of the drug registration process and uses the information provided in the Beclomethasone Dipropionate Monohydrate KDMF to evaluate the safety and efficacy of the drug.
After submitting a Beclomethasone Dipropionate Monohydrate KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Beclomethasone Dipropionate Monohydrate API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Beclomethasone Dipropionate Monohydrate suppliers with KDMF on PharmaCompass.
A Beclomethasone Dipropionate Monohydrate CEP of the European Pharmacopoeia monograph is often referred to as a Beclomethasone Dipropionate Monohydrate Certificate of Suitability (COS). The purpose of a Beclomethasone Dipropionate Monohydrate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Beclomethasone Dipropionate Monohydrate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Beclomethasone Dipropionate Monohydrate to their clients by showing that a Beclomethasone Dipropionate Monohydrate CEP has been issued for it. The manufacturer submits a Beclomethasone Dipropionate Monohydrate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Beclomethasone Dipropionate Monohydrate CEP holder for the record. Additionally, the data presented in the Beclomethasone Dipropionate Monohydrate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Beclomethasone Dipropionate Monohydrate DMF.
A Beclomethasone Dipropionate Monohydrate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Beclomethasone Dipropionate Monohydrate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Beclomethasone Dipropionate Monohydrate suppliers with CEP (COS) on PharmaCompass.
A Beclomethasone Dipropionate Monohydrate written confirmation (Beclomethasone Dipropionate Monohydrate WC) is an official document issued by a regulatory agency to a Beclomethasone Dipropionate Monohydrate manufacturer, verifying that the manufacturing facility of a Beclomethasone Dipropionate Monohydrate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Beclomethasone Dipropionate Monohydrate APIs or Beclomethasone Dipropionate Monohydrate finished pharmaceutical products to another nation, regulatory agencies frequently require a Beclomethasone Dipropionate Monohydrate WC (written confirmation) as part of the regulatory process.
click here to find a list of Beclomethasone Dipropionate Monohydrate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Beclomethasone Dipropionate Monohydrate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Beclomethasone Dipropionate Monohydrate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Beclomethasone Dipropionate Monohydrate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Beclomethasone Dipropionate Monohydrate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Beclomethasone Dipropionate Monohydrate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Beclomethasone Dipropionate Monohydrate suppliers with NDC on PharmaCompass.
Beclomethasone Dipropionate Monohydrate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Beclomethasone Dipropionate Monohydrate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Beclomethasone Dipropionate Monohydrate GMP manufacturer or Beclomethasone Dipropionate Monohydrate GMP API supplier for your needs.
A Beclomethasone Dipropionate Monohydrate CoA (Certificate of Analysis) is a formal document that attests to Beclomethasone Dipropionate Monohydrate's compliance with Beclomethasone Dipropionate Monohydrate specifications and serves as a tool for batch-level quality control.
Beclomethasone Dipropionate Monohydrate CoA mostly includes findings from lab analyses of a specific batch. For each Beclomethasone Dipropionate Monohydrate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Beclomethasone Dipropionate Monohydrate may be tested according to a variety of international standards, such as European Pharmacopoeia (Beclomethasone Dipropionate Monohydrate EP), Beclomethasone Dipropionate Monohydrate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Beclomethasone Dipropionate Monohydrate USP).
