
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
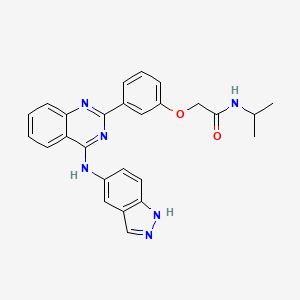

1. Belumosudil
2. Rezurock
1. 911417-87-3
2. Slx-2119
3. Belumosudil
4. Kd-025
5. Kd025
6. Rock Inhibitor 2
7. Rezurock
8. Belumosudil [usan]
9. Slx 2119
10. Slx-2119 Free Base
11. Rock2 Inhibitor Kd025
12. 2-[3-[4-(1h-indazol-5-ylamino)quinazolin-2-yl]phenoxy]-n-propan-2-ylacetamide
13. Slx2119
14. 834yjf89wo
15. C26h24n6o2
16. Kd025 (slx-2119)
17. Acetamide, 2-(3-(4-(1h-indazol-5-ylamino)-2-quinazolinyl)phenoxy)-n-(1-methylethyl)-
18. 2-(3-(4-(1h-indazol-5-ylamino)quinazolin-2-yl)phenoxy)-n-isopropylacetamide
19. 2-[3-[4-(1~{h}-indazol-5-ylamino)quinazolin-2-yl]phenoxy]-~{n}-propan-2-yl-ethanamide
20. 2-[3-[4-(1h-indazol-5-ylamino)-2-quinazolinyl]phenoxy]-n-(1-methylethyl)-acetamide
21. 2-[3-[4-[(1h-indazol-5-yl)amino]quinazolin-2-yl]phenoxy]-n-isopropylacetamide
22. Unii-834yjf89wo
23. Kinome_2597
24. Belumosudil [inn]
25. Belumosudil [who-dd]
26. Schembl878202
27. Gtpl9558
28. Chembl2005186
29. Dtxsid80238425
30. Bdbm322155
31. Bdbm435505
32. Bcp15921
33. Ex-a2314
34. Kd 025
35. Mfcd23098791
36. S7936
37. Who 11343
38. Zinc63298464
39. Slx-2119(kd-025)
40. Slx-2119; Kd-025
41. Us10570123, Example 232
42. Ccg-269256
43. Cs-0776
44. Sb16915
45. Ncgc00378903-01
46. Ncgc00378903-02
47. Ac-33057
48. As-35230
49. Hy-15307
50. Db-103511
51. Us10183931, Slx-2119
52. A855956
53. Q27269397
54. 2-(3-{4-[(1h-indazol-5-yl)amino]quinazolin-2-yl}phenoxy)-n-(propan-2-yl)acetamide
55. 2-[3-[4-(1h-indazol-5-ylamino)-2-quinazolinyl]phenoxy]-n-(1-methylethyl)acetamide
56. Icq
| Molecular Weight | 452.5 g/mol |
|---|---|
| Molecular Formula | C26H24N6O2 |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 452.19607403 g/mol |
| Monoisotopic Mass | 452.19607403 g/mol |
| Topological Polar Surface Area | 105 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 678 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Belumosudil is indicated for the treatment of chronic graft-versus-host disease (GVHD) in adult and pediatric patients 12 years of age and older following failure of at least two other lines of systemic therapy.
Belumosudil appears to inhibit several pro-fibrotic and pro-inflammatory processes in order to prevent and treat the damage incurred by graft-versus-host disease. Given its mechanism of action and findings in animal trials, belumosudil is considered to carry embryo-fetal toxicity and may cause significant harm to a developing fetus should a pregnant mother be exposed. Female patients of reproductive potential, or male patients with female partners of reproductive potential, should be advised to use effective contraception during treatment with belumosudil and for one week after the last dose.
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AA - Selective immunosuppressants
L04AA48 - Belumosudil
Absorption
Following oral administration, the mean bioavailability of belumosudil is 64% and the median Tmax at steady-state is 1.26 to 2.53 hours. As compared to administration in a fasted state, belumosudil Cmax and AUC increased by 2.2 and 2 times, respectively, when administered with a high-fat, high-calorie meal.
Route of Elimination
Belumosudil is eliminated primarily in the feces. Following the administration of a radiolabeled oral dose of belumosudil in healthy subjects, approximately 85% of the radioactivity was recovered in the feces, 30% of which was unchanged parent drug, with less than 5% recovered in the urine.
Volume of Distribution
Following a single oral dose of belumosudil in healthy subjects, the mean geometric volume of distribution was 184 L.
Clearance
The mean clearance of belumosudil is 9.83 L/h.
The _in vitro_ metabolism of belumosudil occurs primarily via CYP3A4 and to a lesser extent by CYP2C8, CYP2D6, and UGT1A9. The specific metabolites generated by belumosudil metabolism remain unclear.
The mean elimination half-life of belumosudil following oral administration is 19 hours.
Chronic graft-versus-host disease (GVHD) is a life-threatening complication of allogeneic hematopoietic stem cell transplantation in which the transplanted donor T-cells recognize the recipient's tissues as foreign and mount an immune response. During the conditioning regimen prior to stem cell transplantation (e.g. involving irradiation or chemotherapy) the host tissues can become damaged which results in downstream inflammatory responses and the generation of inflammatory mediators like TNF-alpha and IL-1. These cytokines increase the expression of host major histocompatibility (MHC) antigens and adhesion molecules which enhances the ability of mature donor T-cells to recognize these molecules. The activation of these donor T-cells results in the activation of mononuclear phagocytes, whose effector functions are triggered by stimulatory molecules generated by the damage incurred during the conditioning phase of treatment. Activated macrophages and cytotoxic T-lymphocytes begin to directly lyse target cells and/or cause their apoptosis, which eventually leads to local tissue damage and further inflammatory responses. Belumosudil is an inhibitor of Rho-associated coiled-coil kinase 2 (ROCK2), a protein that plays a vital role in the pathogenesis of immune and fibrotic diseases. The inhibition of ROCK2 has been shown to resolve immune dysregulation by down-regulating pro-inflammatory Th17 cells and up-regulating regulatory T-cells by manipulating the phosphorylation of STAT3 and STAT5.
GDUFA
DMF Review : Complete
Rev. Date : 2025-02-26
Pay. Date : 2025-01-23
DMF Number : 40514
Submission : 2024-09-29
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Company :
Belumosudil Mesylate
Drug Cost (USD) : 100,434,804
Year : 2022
Prescribers : 797
Prescriptions : 4612

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
Company :
Belumosudil Mesylate
Drug Cost (USD) : 21,356,248
Year : 2021
Prescribers : 368
Prescriptions : 991

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
32
PharmaCompass offers a list of Belumosudil API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Belumosudil manufacturer or Belumosudil supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Belumosudil manufacturer or Belumosudil supplier.
PharmaCompass also assists you with knowing the Belumosudil API Price utilized in the formulation of products. Belumosudil API Price is not always fixed or binding as the Belumosudil Price is obtained through a variety of data sources. The Belumosudil Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Belumosudil Mesylate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Belumosudil Mesylate, including repackagers and relabelers. The FDA regulates Belumosudil Mesylate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Belumosudil Mesylate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Belumosudil Mesylate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Belumosudil Mesylate supplier is an individual or a company that provides Belumosudil Mesylate active pharmaceutical ingredient (API) or Belumosudil Mesylate finished formulations upon request. The Belumosudil Mesylate suppliers may include Belumosudil Mesylate API manufacturers, exporters, distributors and traders.
click here to find a list of Belumosudil Mesylate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Belumosudil Mesylate DMF (Drug Master File) is a document detailing the whole manufacturing process of Belumosudil Mesylate active pharmaceutical ingredient (API) in detail. Different forms of Belumosudil Mesylate DMFs exist exist since differing nations have different regulations, such as Belumosudil Mesylate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Belumosudil Mesylate DMF submitted to regulatory agencies in the US is known as a USDMF. Belumosudil Mesylate USDMF includes data on Belumosudil Mesylate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Belumosudil Mesylate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Belumosudil Mesylate suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Belumosudil Mesylate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Belumosudil Mesylate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Belumosudil Mesylate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Belumosudil Mesylate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Belumosudil Mesylate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Belumosudil Mesylate suppliers with NDC on PharmaCompass.
Belumosudil Mesylate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Belumosudil Mesylate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Belumosudil Mesylate GMP manufacturer or Belumosudil Mesylate GMP API supplier for your needs.
A Belumosudil Mesylate CoA (Certificate of Analysis) is a formal document that attests to Belumosudil Mesylate's compliance with Belumosudil Mesylate specifications and serves as a tool for batch-level quality control.
Belumosudil Mesylate CoA mostly includes findings from lab analyses of a specific batch. For each Belumosudil Mesylate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Belumosudil Mesylate may be tested according to a variety of international standards, such as European Pharmacopoeia (Belumosudil Mesylate EP), Belumosudil Mesylate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Belumosudil Mesylate USP).
