
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
Finished Drug Prices
NA
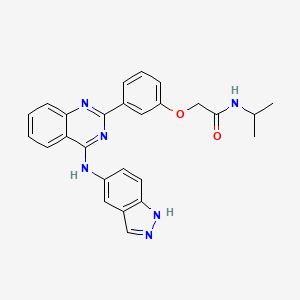

1. Belumosudil
2. Rezurock
1. 911417-87-3
2. Slx-2119
3. Belumosudil
4. Kd-025
5. Kd025
6. Rock Inhibitor 2
7. Rezurock
8. Belumosudil [usan]
9. Slx 2119
10. Slx-2119 Free Base
11. Rock2 Inhibitor Kd025
12. 2-[3-[4-(1h-indazol-5-ylamino)quinazolin-2-yl]phenoxy]-n-propan-2-ylacetamide
13. Slx2119
14. 834yjf89wo
15. C26h24n6o2
16. Kd025 (slx-2119)
17. Acetamide, 2-(3-(4-(1h-indazol-5-ylamino)-2-quinazolinyl)phenoxy)-n-(1-methylethyl)-
18. 2-(3-(4-(1h-indazol-5-ylamino)quinazolin-2-yl)phenoxy)-n-isopropylacetamide
19. 2-[3-[4-(1~{h}-indazol-5-ylamino)quinazolin-2-yl]phenoxy]-~{n}-propan-2-yl-ethanamide
20. 2-[3-[4-(1h-indazol-5-ylamino)-2-quinazolinyl]phenoxy]-n-(1-methylethyl)-acetamide
21. 2-[3-[4-[(1h-indazol-5-yl)amino]quinazolin-2-yl]phenoxy]-n-isopropylacetamide
22. Unii-834yjf89wo
23. Kinome_2597
24. Belumosudil [inn]
25. Belumosudil [who-dd]
26. Schembl878202
27. Gtpl9558
28. Chembl2005186
29. Dtxsid80238425
30. Bdbm322155
31. Bdbm435505
32. Bcp15921
33. Ex-a2314
34. Kd 025
35. Mfcd23098791
36. S7936
37. Who 11343
38. Zinc63298464
39. Slx-2119(kd-025)
40. Slx-2119; Kd-025
41. Us10570123, Example 232
42. Ccg-269256
43. Cs-0776
44. Sb16915
45. Ncgc00378903-01
46. Ncgc00378903-02
47. Ac-33057
48. As-35230
49. Hy-15307
50. Db-103511
51. Us10183931, Slx-2119
52. A855956
53. Q27269397
54. 2-(3-{4-[(1h-indazol-5-yl)amino]quinazolin-2-yl}phenoxy)-n-(propan-2-yl)acetamide
55. 2-[3-[4-(1h-indazol-5-ylamino)-2-quinazolinyl]phenoxy]-n-(1-methylethyl)acetamide
56. Icq
| Molecular Weight | 452.5 g/mol |
|---|---|
| Molecular Formula | C26H24N6O2 |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 452.19607403 g/mol |
| Monoisotopic Mass | 452.19607403 g/mol |
| Topological Polar Surface Area | 105 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 678 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Belumosudil is indicated for the treatment of chronic graft-versus-host disease (GVHD) in adult and pediatric patients 12 years of age and older following failure of at least two other lines of systemic therapy.
Belumosudil appears to inhibit several pro-fibrotic and pro-inflammatory processes in order to prevent and treat the damage incurred by graft-versus-host disease. Given its mechanism of action and findings in animal trials, belumosudil is considered to carry embryo-fetal toxicity and may cause significant harm to a developing fetus should a pregnant mother be exposed. Female patients of reproductive potential, or male patients with female partners of reproductive potential, should be advised to use effective contraception during treatment with belumosudil and for one week after the last dose.
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L - Antineoplastic and immunomodulating agents
L04 - Immunosuppressants
L04A - Immunosuppressants
L04AA - Selective immunosuppressants
L04AA48 - Belumosudil
Absorption
Following oral administration, the mean bioavailability of belumosudil is 64% and the median Tmax at steady-state is 1.26 to 2.53 hours. As compared to administration in a fasted state, belumosudil Cmax and AUC increased by 2.2 and 2 times, respectively, when administered with a high-fat, high-calorie meal.
Route of Elimination
Belumosudil is eliminated primarily in the feces. Following the administration of a radiolabeled oral dose of belumosudil in healthy subjects, approximately 85% of the radioactivity was recovered in the feces, 30% of which was unchanged parent drug, with less than 5% recovered in the urine.
Volume of Distribution
Following a single oral dose of belumosudil in healthy subjects, the mean geometric volume of distribution was 184 L.
Clearance
The mean clearance of belumosudil is 9.83 L/h.
The _in vitro_ metabolism of belumosudil occurs primarily via CYP3A4 and to a lesser extent by CYP2C8, CYP2D6, and UGT1A9. The specific metabolites generated by belumosudil metabolism remain unclear.
The mean elimination half-life of belumosudil following oral administration is 19 hours.
Chronic graft-versus-host disease (GVHD) is a life-threatening complication of allogeneic hematopoietic stem cell transplantation in which the transplanted donor T-cells recognize the recipient's tissues as foreign and mount an immune response. During the conditioning regimen prior to stem cell transplantation (e.g. involving irradiation or chemotherapy) the host tissues can become damaged which results in downstream inflammatory responses and the generation of inflammatory mediators like TNF-alpha and IL-1. These cytokines increase the expression of host major histocompatibility (MHC) antigens and adhesion molecules which enhances the ability of mature donor T-cells to recognize these molecules. The activation of these donor T-cells results in the activation of mononuclear phagocytes, whose effector functions are triggered by stimulatory molecules generated by the damage incurred during the conditioning phase of treatment. Activated macrophages and cytotoxic T-lymphocytes begin to directly lyse target cells and/or cause their apoptosis, which eventually leads to local tissue damage and further inflammatory responses. Belumosudil is an inhibitor of Rho-associated coiled-coil kinase 2 (ROCK2), a protein that plays a vital role in the pathogenesis of immune and fibrotic diseases. The inhibition of ROCK2 has been shown to resolve immune dysregulation by down-regulating pro-inflammatory Th17 cells and up-regulating regulatory T-cells by manipulating the phosphorylation of STAT3 and STAT5.
Global Sales Information

Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?