
Synopsis
0
JDMF
0
KDMF
0
VMF
0
Canada

0
Australia

0
South Africa

0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
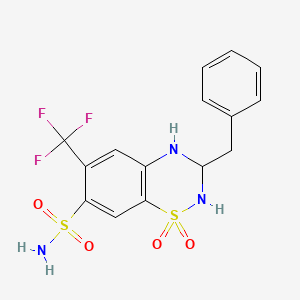

1. Aprinox
2. Bendrofluazide
3. Benzide
4. Benzide M
5. Benzide-m
6. Berkozide
7. Centyl
8. Esberizid
9. Naturetin
10. Naturine
11. Neo Naclex
12. Neo-naclex
13. Pluryl
14. Urizid
1. Bendrofluazide
2. 73-48-3
3. Naturetin
4. Aprinox
5. Benzhydroflumethiazide
6. Benzylrodiuran
7. Berkozide
8. Centyl
9. Pluryl
10. Neo-naclex
11. Bentride
12. Bristuric
13. Bristuron
14. Flumesil
15. Niagaril
16. Plusuril
17. Poliuron
18. Sinesalin
19. Sodiuretic
20. Thiazidico
21. Benuron
22. Intolex
23. Livesan
24. Nikion
25. Orsile
26. Pluryle
27. Repicin
28. Salural
29. Salures
30. Urlea
31. Benzylhydroflumethiazide
32. Neo-rontyl
33. Relan Beta
34. Bhft
35. Rauzide
36. Benzydroflumethiazide
37. Bendrofumethiazide
38. Rautrax N
39. Bendroflumethiazidum
40. Bendroflumethazide
41. Bendroflumetiazida
42. Bendroflumethiazidum [inn-latin]
43. Bendroflumetiazida [inn-spanish]
44. Be 724-a
45. Bl H368
46. 2h-1,2,4-benzothiadiazine-7-sulfonamide, 3,4-dihydro-3-(phenylmethyl)-6-(trifluoromethyl)-, 1,1-dioxide
47. Nsc-758229
48. 5q52x6icji
49. 3-benzyl-3,4-dihydro-6-(trifluoromethyl)-2h-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
50. 6-trifluoromethyl-3-benzyl-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine, 1,1-dioxide
51. Mls000028558
52. Chebi:3013
53. Naturetin-2.5
54. Naturine
55. Flumersil
56. Naigaril
57. Nateretin
58. Smr000058802
59. Bendroflumethiazide-d5
60. Benzy-rodiuran
61. Dsstox_cid_2647
62. Bendroflumetiazide
63. Naturetin-5
64. Naturetin-10
65. 6-trifluoromethyl-3-benzyl-7-sulfamyl-3,4-dihydro-1,2,4-benzothiadiazine 1,1-dioxide
66. Dsstox_rid_76673
67. Dsstox_gsid_22647
68. 3-benzyl-1,1-dioxo-6-(trifluoromethyl)-3,4-dihydro-2h-1lambda6,2,4-benzothiadiazine-7-sulfonamide
69. Bendroflumetiazide [dcit]
70. Ft 81
71. 3-benzyl-1,1-dioxo-6-(trifluoromethyl)-3,4-dihydro-2h-1,2,4- Benzothiadiazine-7-sulfonamide
72. Naturetin (tn)
73. Hsdb 3293
74. Sr-01000000103
75. Einecs 200-800-1
76. Bendroflumethiazide [inn]
77. Unii-5q52x6icji
78. Brn 0373316
79. Neonaclex
80. Cas-73-48-3
81. Relan .beta.
82. Ncgc00016312-01
83. 3-benzyl-1,1-dioxo-6-(trifluoromethyl)-3,4-dihydro-2h-1?^{6},2,4-benzothiadiazine-7-sulfonamide
84. Bendroflumethiazide (jan/usp/inn)
85. Prestwick_992
86. Behenic Fatty Acids
87. 2h-1,2,4-benzothiadiazine-7-sulfonamide, 3-benzyl-3,4-dihydro-6-(trifluoromethyl)-, 1,1-dioxide
88. 3-benzyl-6-trifluoromethyl-7-sulfamoyl-3,4-dihydro-1,2,4-benzothiadiazine, 1,1-dioxide
89. Bendroflumethiazide [usp:inn:ban:jan]
90. Spectrum_001386
91. Opera_id_1043
92. Prestwick0_000784
93. Prestwick1_000784
94. Prestwick2_000784
95. Prestwick3_000784
96. Spectrum2_001524
97. Spectrum3_001558
98. Spectrum4_000774
99. Spectrum5_001016
100. 3-benzyl-6-(trifluoromethyl)-3,4-dihydro-2h-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
101. Chembl1684
102. Schembl26016
103. Bspbio_000888
104. Bspbio_003036
105. Kbiogr_001188
106. Kbioss_001866
107. 2h-1,2,4-benzothiadiazine-7-sulfonamide, 3-benzyl-3,4-dihydro-6-(trifluoromethyl)-,1,1-dioxide
108. 4-27-00-08041 (beilstein Handbook Reference)
109. Mls001076062
110. Mls002548857
111. Divk1c_000274
112. Spectrum1503104
113. Spbio_001308
114. Spbio_002827
115. (.+/-.)-bendroflumethiazide
116. Bpbio1_000978
117. Gtpl7122
118. Bendroflumethiazide [mi]
119. Dtxsid5022647
120. Bendroflumethiazide [jan]
121. Hms500n16
122. Kbio1_000274
123. Kbio2_001866
124. Kbio2_004434
125. Kbio2_007002
126. Kbio3_002536
127. Bendroflumethiazide [hsdb]
128. Ninds_000274
129. Bendroflumethiazide [vandf]
130. Hms1570m10
131. Hms1922e15
132. Hms2092j16
133. Hms2097m10
134. Hms2230g10
135. Hms3259d17
136. Hms3370i20
137. Hms3714m10
138. Pharmakon1600-01503104
139. Bendroflumethiazide [mart.]
140. Bcp24525
141. Hy-b1363
142. Bendroflumethiazide [usp-rs]
143. Bendroflumethiazide [who-dd]
144. Tox21_110365
145. Ccg-39302
146. Mfcd00078963
147. Nsc758229
148. Akos024255718
149. Tox21_110365_1
150. Bendroflumethiazide, Analytical Standard
151. Db00436
152. Hs-0095
153. Nc00664
154. Nsc 758229
155. 3-benzyl-6-trifluoromethyl-7-sulfamoyl-3,4-dihydro-2h-1,2,4-benzothiadiazine 1,1-dioxide
156. Idi1_000274
157. Bendroflumethiazide [ep Impurity]
158. Bendroflumethiazide [orange Book]
159. Ncgc00018194-02
160. Ncgc00018194-03
161. Ncgc00018194-04
162. Ncgc00018194-08
163. Ncgc00089729-02
164. Ncgc00089729-03
165. (+-)-3-benzyl-3,4-dihydro-6-(trifluoromethyl)-2h-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
166. 2h-1,2,4-benzothiadiazine-7-sulfonamide, 3,4-dihydro-3-(phenylmethyl)-6-(trifluoromethyl)-, 1,1-dioxide, (+-)-
167. 3,4-dihydro-3-(phenylmethyl)-6-(trifluoromethyl)-2h-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
168. 3-(phenylmethyl)-6-(trifluoromethyl)-3,4-dihydro-2h-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
169. 3-benzyl-1,1-dioxo-6-(trifluoromethyl)-3,4-dihydro-2h-1$l^{6},2,4-benzothiadiazine-7-sulfonamide
170. Bendroflumethiazide [ep Monograph]
171. Bendroflumethiazide [usp Monograph]
172. Sbi-0051772.p002
173. Corzide Component Bendroflumethiazide
174. Ab00052315
175. Cs-0013104
176. Eu-0009291
177. Ft-0662518
178. Ft-0662519
179. Ft-0662520
180. Sw028069-4
181. Unm000000601501
182. Bendroflumethiazide Component Of Corzide
183. C07758
184. D00650
185. Ab00052315_15
186. Ab00052315_16
187. 073b483
188. A851258
189. Q1169164
190. Sr-01000000103-2
191. Sr-01000000103-4
192. Brd-a80017228-001-05-9
193. Brd-a80017228-001-15-8
194. Z2786051551
195. Bendroflumethiazide, British Pharmacopoeia (bp) Reference Standard
196. Bendroflumethiazide, European Pharmacopoeia (ep) Reference Standard
197. Bendroflumethiazide, United States Pharmacopeia (usp) Reference Standard
198. 3-benzyl-6-trifluoromethyl-7-sulfamoyl-3,4-dihydro-1,2,4-benzothiadiazine 1,1-dioxide
199. (+/-)-3-benzyl-3,4-dihydro-6-(trifluoromethyl)-2h-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide
200. 2h-1,2,4-benzothiadiazine-7-sulfonamide, 3,4-dihydro-3-(phenylmethyl)-6-(trifluoromethyl)-, 1,1-dioxide, (+/-)-
201. 3,4-dihydro-3-(phenylmethyl)-6-(trifluoromethyl)-2h-1,2,4-benzothiadiazine-7-sulfonamide-1,1-dioxide
202. 3-benzyl-6-(trifluoromethyl)-3,4-dihydro-2h-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide #
203. 3-benzyl-6-(trifluoromethyl)-3,4-dihydro-2h-benzo[e][1,2,4]thiadiazine-7-sulfonamide 1,1-dioxide
| Molecular Weight | 421.4 g/mol |
|---|---|
| Molecular Formula | C15H14F3N3O4S2 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 3 |
| Exact Mass | 421.03778277 g/mol |
| Monoisotopic Mass | 421.03778277 g/mol |
| Topological Polar Surface Area | 135 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 740 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Corzide |
| PubMed Health | Nadolol/Bendroflumethiazide (By mouth) |
| Drug Classes | Beta-Adrenergic Blocker, Nonselective/Thiazide Combination |
| Active Ingredient | nadolol; Bendroflumethiazide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 80mg; 40mg |
| Market Status | Prescription |
| Company | King Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Corzide |
| PubMed Health | Nadolol/Bendroflumethiazide (By mouth) |
| Drug Classes | Beta-Adrenergic Blocker, Nonselective/Thiazide Combination |
| Active Ingredient | nadolol; Bendroflumethiazide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 5mg; 80mg; 40mg |
| Market Status | Prescription |
| Company | King Pharms |
Antihypertensive Agents; Diuretics, Thiazide
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
IT IS INDICATED IN CONTROL OF EDEMA, CONGESTIVE HEART FAILURE, NEPHROSIS & NEPHRITIS, CIRRHOSIS & ASCITES, & OTHER EDEMATOUS STATES. ... IT IS ALSO OF VALUE IN HYPERTENSION ALONE OR WHEN COMBINED WITH OTHER ANTIHYPERTENSIVES.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 868
THIAZIDE DRUGS...USUALLY FIRST DRUG TO BE EMPLOYED IN TREATMENT OF HYPERTENSION. SINCE THIAZIDES INDUCE ONLY LIMITED (10%) REDN IN BLOOD PRESSURE THEY ARE USEFUL EITHER IN MILD CASES OF HYPERTENSION OR AS ADJUNCTIVE THERAPY TO OTHER DRUGS. /THIAZIDE DIURETICS/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 868
THIAZIDE DIURETICS ARE EFFECTIVE AS ADJUNCTIVE THERAPY IN EDEMA ASSOC WITH CONGESTIVE HEART FAILURE, HEPATIC CIRRHOSIS, & CORTICOSTEROID & ESTROGEN THERAPY, AS WELL AS EDEMA DUE TO VARIOUS FORMS OF RENAL DYSFUNCTION...& SEVERE EDEMA DUE TO PREGNANCY. /THIAZIDE DIURETICS/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 868
For more Therapeutic Uses (Complete) data for BENDROFLUMETHIAZIDE (10 total), please visit the HSDB record page.
PERIODIC SERUM ELECTROLYTE DETERMINATION SHOULD BE DONE ON ALL PT IN ORDER TO DETECT ELECTROLYTE IMBALANCE SUCH AS HYPONATREMIA, HYPOCHLOREMIC ALKALOSIS, & HYPOKALEMIA. /THIAZIDE DIURETICS/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 868
THIAZIDE DIURETICS ARE CONTRAINDICATED IN ANURIA, PATIENTS HYPERSENSITIVE TO THESE & OTHER SULFONAMIDE DRUGS, & IN OTHERWISE HEALTHY PREGNANT WOMEN WITH OR WITHOUT MILD EDEMA. ...SHOULD BE USED WITH CAUTION IN PATIENTS WITH RENAL DISEASE, SINCE THEY MAY PPT AZOTEMIA. /THIAZIDE DIURETICS/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 868
PLASMA POTASSIUM CONCN SHOULD BE DETERMINED PERIODICALLY IN PT WHO RECEIVE THIAZIDE DIURETICS FOR EXTENDED PERIODS. /THIAZIDE DIURETICS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 832
Maternal Medication usually Compatible with Breast-Feeding: Bendroflumethiazide: Reported Sign or Symptom in Infant or Effect on Lactation: Suppresses lactation. /From Table 6/
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 140 (1994)
For more Drug Warnings (Complete) data for BENDROFLUMETHIAZIDE (20 total), please visit the HSDB record page.
3. 3= MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 0.5-5 G/KG, BETWEEN 1 OZ & 1 PINT FOR 70 KG PERSON (150 LB). /BENZOTHIADIAZIDE DIURETICS/
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-239
For the treatment of high blood pressure and management of edema related to heart failure.
Bendroflumethiazide, a thiazide diuretic, removes excess water from the body by increasing how often you urinate (pass water) and also widens the blood vessels which helps to reduce blood pressure. It inhibits Na+/Cl- reabsorption from the distal convoluted tubules in the kidneys. Thiazides also cause loss of potassium and an increase in serum uric acid. Thiazides are often used to treat hypertension, but their hypotensive effects are not necessarily due to their diuretic activity. Thiazides have been shown to prevent hypertension-related morbidity and mortality although the mechanism is not fully understood. Thiazides cause vasodilation by activating calcium-activated potassium channels (large conductance) in vascular smooth muscles and inhibiting various carbonic anhydrases in vascular tissue.
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Diuretics
Agents that promote the excretion of urine through their effects on kidney function. (See all compounds classified as Diuretics.)
Sodium Chloride Symporter Inhibitors
Agents that inhibit SODIUM CHLORIDE SYMPORTERS. They act as DIURETICS. Excess use is associated with HYPOKALEMIA. (See all compounds classified as Sodium Chloride Symporter Inhibitors.)
C - Cardiovascular system
C03 - Diuretics
C03A - Low-ceiling diuretics, thiazides
C03AA - Thiazides, plain
C03AA01 - Bendroflumethiazide
Absorption
Absorbed relatively rapidly after oral administration
MOST CMPD ARE RAPIDLY EXCRETED WITHIN 3-6 HR. BENDROFLUMETHIAZIDE...LONGER DURATION OF ACTION THAT IS CORRELATED WITH...SLOWER EXCRETION.
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 902
THIAZIDES ARE ABSORBED FROM GI TRACT & OWE THEIR USEFULNESS LARGELY TO THEIR EFFECTIVENESS BY ORAL ROUTE. ABSORPTION IS RELATIVELY RAPID. MOST AGENTS SHOW DEMONSTRABLE DIURETIC EFFECT WITHIN HR AFTER ORAL ADMIN. /BENZOTHIADIAZIDES/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 831
IN GENERAL, THIAZIDES WITH RELATIVELY LONG DURATIONS OF ACTION SHOW PROPORTIONATELY HIGH DEGREE OF BINDING TO PLASMA PROTEINS & ARE REABSORBED... BY RENAL TUBULES. ... DRUG PASSES READILY THROUGH PLACENTAL BARRIER TO FETUS. ALL THIAZIDES PROBABLY UNDERGO ACTIVE SECRETION IN PROXIMAL TUBULE. /THIAZIDE DIURETICS/
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 902
BENDROFLUMETHIAZIDE WAS ADMIN ORALLY TO 9 HEALTHY VOLUNTEERS. PEAK PLASMA LEVELS REACHED @ 1 + OR - 0.4 HR. CONCN DECLINED WITH MEAN T/2 OF 3 HR. APPARENT VOL OF DISTRIBUTION AVG 1.48 L/KG. MAJOR PART ELIMINATED VIA NONRENAL MECHANISMS. URINARY RECOVERY AVG 30%.
BEERMANN B ET AL; CLIN PHARMACOL THER 22(OCT) 385 (1977)
Bendroflumethiazide appears to be well absorbed from the GI tract. The drug is excreted unchanged in urine, and excretion is essentially complete within 24 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 2166
8.5 hours
Halflife is 3-3.9 hrs. /From table/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 703
As a diuretic, bendroflumethiazide inhibits active chloride reabsorption at the early distal tubule via the Na-Cl cotransporter, resulting in an increase in the excretion of sodium, chloride, and water. Thiazides like bendroflumethiazide also inhibit sodium ion transport across the renal tubular epithelium through binding to the thiazide sensitive sodium-chloride transporter. This results in an increase in potassium excretion via the sodium-potassium exchange mechanism. The antihypertensive mechanism of bendroflumethiazide is less well understood although it may be mediated through its action on carbonic anhydrases in the smooth muscle or through its action on the large-conductance calcium-activated potassium (KCa) channel, also found in the smooth muscle.
...BENZOTHIADIAZIDES HAVE DIRECT EFFECT ON RENAL TUBULAR TRANSPORT OF SODIUM & CHLORIDE...INDEPENDENT OF ANY EFFECT ON CARBONIC ANHYDRASE. /THIAZIDE DIURETICS/
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 899
NATURE OF CHEM INTERACTION BETWEEN THIAZIDES & SPECIFIC RENAL RECEPTORS RESPONSIBLE FOR CHLORURETIC EFFECT IS NOT KNOWN; NO CRITICAL ENZYMATIC REACTIONS HAVE BEEN IDENTIFIED. /THIAZIDE DIURETICS/
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 901
...MAY DECR EXCRETION OF URIC ACID IN MAN, THUS INCR ITS CONCN IN PLASMA. HYPERURICEMIC EFFECT RESULTS PRIMARILY FROM INHIBITION OF TUBULAR SECRETION OF URATE. ... UNLIKE MOST OTHER NATRIURETIC AGENTS...DECR RENAL EXCRETION OF CALCIUM RELATIVE TO THAT OF SODIUM... /ENHANCE/ EXCRETION OF MAGNESIUM... /THIAZIDE DIURETICS/
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 901
THIAZIDES INHIBIT REABSORPTION OF SODIUM &...CHLORIDE IN DISTAL SEGMENT. ... AS CLASS...HAVE IMPORTANT ACTION ON EXCRETION OF POTASSIUM THAT RESULTS FROM INCR SECRETION OF CATION BY DISTAL TUBULE. ... GLOMERULAR FILTRATION RATE MAY BE REDUCED BY THIAZIDES, PARTICULARLY WITH IV ADMIN FOR EXPTL PURPOSES. /THIAZIDE DIURETICS/
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 901
For more Mechanism of Action (Complete) data for BENDROFLUMETHIAZIDE (11 total), please visit the HSDB record page.
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 15847
Submission : 2002-02-04
Status : Active
Type : II

Certificate Number : CEP 2002-102 - Rev 02
Issue Date : 2024-11-05
Type : Chemical
Substance Number : 370
Status : Valid

Date of Issue : 2022-06-30
Valid Till : 2025-07-28
Written Confirmation Number : WC-0063
Address of the Firm :

NDC Package Code : 53747-004
Start Marketing Date : 2002-02-04
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 19857
Submission : 2006-11-07
Status : Inactive
Type : II

Certificate Number : R1-CEP 2007-142 - Rev 03
Issue Date : 2018-04-23
Type : Chemical
Substance Number : 370
Status : Valid

Date of Issue : 2022-06-08
Valid Till : 2025-07-02
Written Confirmation Number : WC-0091
Address of the Firm :



NDC Package Code : 69988-0028
Start Marketing Date : 2008-12-01
End Marketing Date : 2025-12-31
Dosage Form (Strength) : POWDER (1kg/1)
Marketing Category : DRUG FOR FURTHER PROCESSING

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
80
PharmaCompass offers a list of Bendroflumethiazide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Bendroflumethiazide manufacturer or Bendroflumethiazide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Bendroflumethiazide manufacturer or Bendroflumethiazide supplier.
PharmaCompass also assists you with knowing the Bendroflumethiazide API Price utilized in the formulation of products. Bendroflumethiazide API Price is not always fixed or binding as the Bendroflumethiazide Price is obtained through a variety of data sources. The Bendroflumethiazide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Bendroflumethiazide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Bendroflumethiazide, including repackagers and relabelers. The FDA regulates Bendroflumethiazide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Bendroflumethiazide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Bendroflumethiazide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Bendroflumethiazide supplier is an individual or a company that provides Bendroflumethiazide active pharmaceutical ingredient (API) or Bendroflumethiazide finished formulations upon request. The Bendroflumethiazide suppliers may include Bendroflumethiazide API manufacturers, exporters, distributors and traders.
click here to find a list of Bendroflumethiazide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Bendroflumethiazide DMF (Drug Master File) is a document detailing the whole manufacturing process of Bendroflumethiazide active pharmaceutical ingredient (API) in detail. Different forms of Bendroflumethiazide DMFs exist exist since differing nations have different regulations, such as Bendroflumethiazide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Bendroflumethiazide DMF submitted to regulatory agencies in the US is known as a USDMF. Bendroflumethiazide USDMF includes data on Bendroflumethiazide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Bendroflumethiazide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Bendroflumethiazide suppliers with USDMF on PharmaCompass.
A Bendroflumethiazide CEP of the European Pharmacopoeia monograph is often referred to as a Bendroflumethiazide Certificate of Suitability (COS). The purpose of a Bendroflumethiazide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Bendroflumethiazide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Bendroflumethiazide to their clients by showing that a Bendroflumethiazide CEP has been issued for it. The manufacturer submits a Bendroflumethiazide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Bendroflumethiazide CEP holder for the record. Additionally, the data presented in the Bendroflumethiazide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Bendroflumethiazide DMF.
A Bendroflumethiazide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Bendroflumethiazide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Bendroflumethiazide suppliers with CEP (COS) on PharmaCompass.
A Bendroflumethiazide written confirmation (Bendroflumethiazide WC) is an official document issued by a regulatory agency to a Bendroflumethiazide manufacturer, verifying that the manufacturing facility of a Bendroflumethiazide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Bendroflumethiazide APIs or Bendroflumethiazide finished pharmaceutical products to another nation, regulatory agencies frequently require a Bendroflumethiazide WC (written confirmation) as part of the regulatory process.
click here to find a list of Bendroflumethiazide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Bendroflumethiazide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Bendroflumethiazide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Bendroflumethiazide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Bendroflumethiazide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Bendroflumethiazide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Bendroflumethiazide suppliers with NDC on PharmaCompass.
Bendroflumethiazide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Bendroflumethiazide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Bendroflumethiazide GMP manufacturer or Bendroflumethiazide GMP API supplier for your needs.
A Bendroflumethiazide CoA (Certificate of Analysis) is a formal document that attests to Bendroflumethiazide's compliance with Bendroflumethiazide specifications and serves as a tool for batch-level quality control.
Bendroflumethiazide CoA mostly includes findings from lab analyses of a specific batch. For each Bendroflumethiazide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Bendroflumethiazide may be tested according to a variety of international standards, such as European Pharmacopoeia (Bendroflumethiazide EP), Bendroflumethiazide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Bendroflumethiazide USP).
