
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
API
0
FDF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
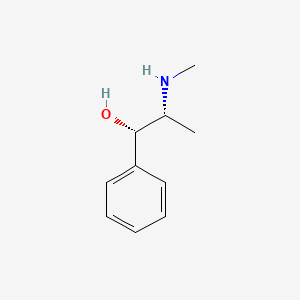

1. (+)-ephedrine
2. L-(+)-ephedrine
3. Racephedrine
4. 321-98-2
5. 90-81-3
6. Ephedrine, (+)-
7. (1s,2r)-ephedrine
8. (1s,2r)-2-(methylamino)-1-phenylpropan-1-ol
9. 03vry66076
10. 72087nsy56
11. ( Inverted Exclamation Marka)-ephedrine
12. (+)-ephedrin
13. (+)-(1s,2r)-ephedrine
14. Ephedrine Dl-form
15. (+)-ephedrine Anhydrous
16. Benzenemethanol, .alpha.-[(1r)-1-(methylamino)ethyl]-, (.alpha.s)-rel-
17. 2-methylamino-1-phenylpropan-1-ol
18. (+-)-ephedrine
19. (1r,2s)-(-)-ephedrine
20. Einecs 206-293-3
21. Racephedrine [inn:ban]
22. Brn 4231286
23. Unii-03vry66076
24. Unii-72087nsy56
25. .psi.-ephedrine
26. .psi.-ephedrin
27. Benzenemethanol, .alpha.-((1r)-1-(methylamino)ethyl)-, (.alpha.s)-rel-
28. Einecs 202-017-0
29. D-.psi.-ephedrine
30. D-(-)-ephedrine
31. (+/-)-ephedrine
32. Novafed (salt/mix)
33. Sinufed (salt/mix)
34. Brn 3197916
35. Tussaphed (salt/mix)
36. (+)-erythro-ephedrine
37. Ephedrine, D-(-)-
38. Symptom 2 (salt/mix)
39. Benzenemethanol, Alpha-((1r)-1-(methylamino)ethyl)-, (alphas)-
40. Benzenemethanol, Alpha-(1-(methylamino)ethyl)-, (s-(r*,s*))-
41. L(+)-.psi.-ephedrine
42. Racephedrine [inn]
43. .psi.-ephedrine, (+)-
44. (1s,2r)-(-)-ephedrine
45. 4-13-00-01881 (beilstein Handbook Reference)
46. Racephedrine [who-dd]
47. Schembl420779
48. Ephedrine, (+-)-
49. Benzenemethanol, .alpha.-[1-(methylamino)ethyl]-, [s-(r*,r*)]-
50. Ephedrine Dl-form [mi]
51. Chembl2110656
52. Niosh/kb0650000
53. Hy-b0980a
54. Zinc74840
55. Alpha-(1-(methylaminoethyl)benzenemethanol, (s-(r*,s*)-
56. Dtxsid20891194
57. Dtxsid60889333
58. Benzenemethanol, .alpha.-[(1r)-1-(methylamino)ethyl]-, (.alpha.s)-
59. Pdsp1_001345
60. Pdsp2_001329
61. Pdsp2_001331
62. Akos027326694
63. Cs-4471
64. Kb06500000
65. 2-(methylamino)-1-phenyl-1-propanol, D-.psi.- #
66. Q22330463
67. (r*,s*)-(+-)-alpha-(1-(methylamino)ethyl)benzyl Alcohol
68. [s-(r*,r*)]-.alpha.-[1-(methylamino)ethyl]benzenemethanol
69. .alpha.-((1s)-1-(methylamino)ethyl)benzyl Alcohol, (.alpha.s)-
70. .alpha.-[(1s)-1-(methylamino)ethyl]benzenemethanol, (.alpha.s)-
71. Benzenemethanol, Alpha-(1-(methylamino)ethyl)-, (r*,s*)-(+-)-
72. Benzenemethanol, .alpha.-(1-(methylamino)ethyl)-, (s-(r*,s*))-
73. Benzenemethanol, Alpha-((1r)-1-(methylamino)ethyl)-, (alphas)-rel-
74. Benzenemethanol, .alpha.-(1-(methylamino)ethyl)-, (r*,s*)-(+/-)-
| Molecular Weight | 165.23 g/mol |
|---|---|
| Molecular Formula | C10H15NO |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 165.115364102 g/mol |
| Monoisotopic Mass | 165.115364102 g/mol |
| Topological Polar Surface Area | 32.3 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 121 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Regulatory Info :
Registration Country : Italy
Brand Name : Rinovit
Dosage Form :
Dosage Strength : Low Dosage Rinol Gtt 30 Ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

SIT LABORATORIO FARMAC. Srl
Dosage Form :
Dosage Strength : Low Dosage Rinol Gtt 30 Ml
Price Per Pack (Euro) : 6.1
Published in :
Country : Italy
RX/OTC/DISCN : Class C

Portfolio PDF
Product Web Link
Virtual Booth
Digital Content
Website
Corporate PDF
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?