
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Europe

0
Australia

0
South Africa

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Bellacarotin
2. Beta Carotene
3. Betacarotene
4. Carotaben
5. Carotene, Beta
6. Max Caro
7. Max-caro
8. Maxcaro
9. Provatene
10. Solatene
11. Vetoron
1. 7235-40-7
2. Beta Carotene
3. Beta,beta-carotene
4. Betacarotene
5. Solatene
6. Provitamin A
7. Carotaben
8. Provatene
9. Serlabo
10. All-trans-beta-carotene
11. Food Orange 5
12. Kpmk
13. Lucaratin
14. Betavit
15. Natural Yellow 26
16. Karotin
17. Provatenol
18. Beta-karotin
19. B-carotene
20. B,b-carotene
21. .beta.-carotene
22. C.i. Food Orange 5
23. .beta.,.beta.-carotene
24. Zlut Prirodni 26
25. All-trans-.beta.-carotene
26. Ci Food Orange 5
27. Carotene,beta
28. Beta-carotin
29. Beta -carotene
30. Beta;-carotene
31. Ci 40800
32. Ci 75130
33. .beta. Carotene
34. Lucarotin 30sun
35. C.i. 75130
36. Nsc 62794
37. Beta, Beta-carotene
38. Betacarotene [inn]
39. (all-e)-1,1'-(3,7,12,16-tetramethyl-1,3,5,7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis(2,6,6-trimethylcyclohexene)
40. 1,3,3-trimethyl-2-[(1e,3e,5e,7e,9e,11e,13e,15e,17e)-3,7,12,16-tetramethyl-18-(2,6,6-trimethylcyclohexen-1-yl)octadeca-1,3,5,7,9,11,13,15,17-nonaenyl]cyclohexene
41. 116-32-5
42. Ins-160a(iii)
43. Ins No.160a(iii)
44. Caroten Base 35468
45. Rovimix .beta.-carotene
46. E-160a(iii)
47. Mls001066383
48. 01yae03m7j
49. 2,2'-((1e,3e,5e,7e,9e,11e,13e,15e,17e)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl)bis(1,3,3-trimethylcyclohex-1-ene)
50. Chebi:17579
51. Nsc62794
52. Betacarotene (inn)
53. Nsc-62794
54. 1,1'-(3,7,12,16-tetramethyl-1,3,5,7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis(2,6,6-trimethylcyclohexene), (all E)-
55. Ncgc00096081-01
56. Smr000112037
57. C.i.-40800
58. Solatene (caps)
59. Karotin [czech]
60. Cyclohexene, 1,1'-(3,7,12,16-tetramethyl-1,3,5,7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis(2,6,6-trimethyl-, (all-e)-
61. .beta.-carotene, All-trans-
62. Betacarotenum [latin]
63. Beta Carotene [usan]
64. Betacaroteno
65. Betacaroteno [spanish]
66. Betacarotenum
67. Mfcd00001556
68. Beta-carotene, All-trans-
69. Betacarotenum [inn-latin]
70. Betacaroteno [inn-spanish]
71. Zlut Prirodni 26 [czech]
72. 1,1'-[(1e,3e,5e,7e,9e,11e,13e,15e,17e)-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaene-1,18-diyl]bis(2,6,6-trimethylcyclohexene)
73. Trans-beta-carotene
74. Ccris 3245
75. Hsdb 3264
76. Diet,beta-carotene Supplementation
77. E160a
78. Sr-01000763803
79. Einecs 230-636-6
80. Beta Carotene [usan:usp]
81. Unii-01yae03m7j
82. Lucarotin
83. Lurotin
84. Beta-carotine
85. All-e-b-carotene
86. Rovimix B-carotene
87. Carotene, .beta.
88. Solatene (tn)
89. Cyclohexene, 1,1'-(3,7,12,16-tetramethyl-1,3,5,7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis[2,6,6-trimethyl-, (all-e)-
90. I(2)-carotene
91. Carotene Base 80s
92. All-trans-b-carotene
93. Beta Carotene Natural
94. Beta Carotene (usp)
95. Trans-.beta.-carotene
96. Carotene, Provitamin A
97. All Trans Beta-carotene
98. B-carotene - 30%
99. All-e-.beta.-carotene
100. All-epsilon-beta-carotene
101. Dsstox_cid_253
102. Spectrum5_000505
103. .beta., .beta.-carotene
104. Bmse000832
105. Ec 230-636-6
106. C40h56 (beta-carotene)
107. (9z,13z)-beta-carotene
108. Beta Carotene [dsc]
109. Beta-carotene [fcc]
110. Chembl1293
111. Dsstox_rid_75466
112. Bidd:pxr0110
113. Dsstox_gsid_20253
114. Beta Carotene [hsdb]
115. Beta-carotene [inci]
116. Betacarotene [mart.]
117. Bspbio_003404
118. Beta Carotene [vandf]
119. Betacarotene [who-dd]
120. Carotene,beta [vandf]
121. .beta.-carotene [mi]
122. Beta Carotene [usp-rs]
123. Dtxsid3020253
124. Bdbm54988
125. Cid_5280489
126. Hms501a12
127. .beta.,.beta.-carotene, Neo B
128. Betacarotene [ep Impurity]
129. Ci 40800 [inci]
130. Ci 75130 [inci]
131. Hms2091m17
132. Pharmakon1600-01500143
133. Betacarotene [ep Monograph]
134. Beta Carotene [orange Book]
135. Hy-n0411
136. Zinc6845076
137. Beta-carotene, >=97.0% (uv)
138. Tox21_111557
139. Ccg-36062
140. Lmpr01070001
141. Nsc755910
142. S1767
143. Beta Carotene [usp Monograph]
144. Akos015896682
145. Ac-1869
146. Db06755
147. Nsc-755910
148. Sdccgmls-0066579.p001
149. Idi1_000330
150. Ncgc00096081-02
151. As-13354
152. Xc175229
153. Cas-7235-40-7
154. Sbi-0051295.p003
155. N1547
156. Sw220035-1
157. C02094
158. D03101
159. Ab00051925_06
160. Ab00051925_07
161. Beta-carotene, Vetec(tm) Reagent Grade, >=93%
162. Q306135
163. Q-200706
164. Sr-01000763803-2
165. Sr-01000763803-3
166. Sr-01000763803-4
167. Beta-carotene (constituent Of Spirulina) [dsc]
168. Beta-carotene, Type I, Synthetic, >=93% (uv), Powder
169. 89648336-f9b2-44a0-9bf8-62e73369cb9b
170. Beta Carotene, United States Pharmacopeia (usp) Reference Standard
171. Beta-carotene, Type Ii, Synthetic, >=95% (hplc), Crystalline
172. Beta-carotene (constituent Of Lycopene And Tomato Extract Containing Lycopene) [dsc]
173. Beta-carotene, Pharmaceutical Secondary Standard; Certified Reference Material
174. Cyclohexane,1,1'-(3,7,12,16-tetramethyl-1,18-octadecanediyl)bis[2,2,6-trimethyl-
175. (all-e)-1,1'-(3,7,12,16-tetramethyl-1,3,5, 7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis[2,6, 6-trimethylcyclohexene]
176. (all-e)-1,1'-(3,7,12,16-tetramethyl-1,3,5,7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis
177. (all-e)-1,1'-(3,7,12,16-tetramethyl-1,3,5,7,9,11,13,15,17-octadecanonaene-1,18-diyl)bis[2,6,6-trimethyl-cyclohexene
178. 1,18-bis(2,6,6-trimethyl-1-cyclohexenyl)-3,7,12,16-tetramethyl-1,3,5,7,9,11,13,15,17-octadecanonene
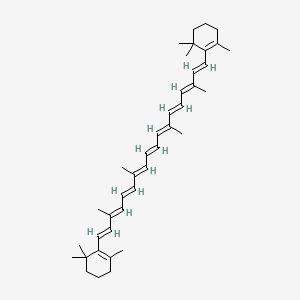
| Molecular Weight | 536.9 g/mol |
|---|---|
| Molecular Formula | C40H56 |
| XLogP3 | 13.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 10 |
| Exact Mass | 536.438201786 g/mol |
| Monoisotopic Mass | 536.438201786 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 1120 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 9 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antioxidants
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
THERAPY WITH ORAL BETA-CAROTENE IN PATIENT WITH POLYMORPHOUS LIGHT ERUPTION; COMPLETE REMISSION OCCURRED IN 32% (6/19) TREATED WITH BETA-CAROTENE.
PMID:427019 PARRISH JA ET AL; BR J DERMATOL 100 (2): 187 (1979)
MEDICATION (VET): VITAMIN A PRECURSOR FOR ALL SPECIES EXCEPT CATS.
Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 282
The effects of chronic oral administration of beta-carotene, a carotenoid partially metabolized to retinol, on plasma lipid concentrations have not been well studied; therefore, 61 subjects were studied over 12 mo while they were enrolled in a skin cancer prevention study in which patients were randomly assigned to receive either placebo (n = 30) or 50 mg beta-carotene/day orally (n = 31). At study entry and 1 yr later, fasting blood samples were obtained for measurement of triglycerides, total cholesterol, high density lipoprotein cholesterol, retinol, and beta-carotene. Retinol concentrations changed minimally in both groups; beta-carotene concentration increased an average of 12.1 + or - 47 nmol/L in the placebo group and 4279 + or - 657 nmol/l in the active treatment group. Both groups experienced similar small increases in triglyceride and total cholesterol concentrations and small decreases in high density lipoprotein cholesterol. Daily oral administration of 50 mg beta-carotene/day did not affect plasma lipid concentrations.
PMID:2000818 Nierenberg DW et al; Am J Clin Nutr 53 (3): 652-4 (1991)
For more Therapeutic Uses (Complete) data for BETA-CAROTENE (10 total), please visit the HSDB record page.
NOT EFFECTIVE AS SUNSCREEN IN NORMAL INDIVIDUALS & SHOULD NOT BE USED FOR THAT PURPOSE ... USED WITH CAUTION IN PT WITH IMPAIRED RENAL OR HEPATIC FUNCTION BECAUSE SAFE USE ... HAS NOT BEEN ESTABLISHED.
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1976
Beta carotene is well tolerated. Carotenodermia is usually the only adverse effect. Patients should be forewarned that carotenodermia will develop after 2-6 weeks of therapy, usually first noticed as yellowness of the palms of the hands or soles of the feet and to a lesser extent of the face. Some patients may experience loose stools during beta carotene therapy, but this is sporadic and may not require discontinuance of therapy. Ecchymoses and arthralgia have been reported rarely
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3555
Beta carotene should be used with caution in patients with impaired renal or hepatic function because safe use of the drug in the presence of these conditions has not been established. Although abnormally high blood concentrations of vitamin A do not occur during beta carotene therapy, patients receiving beta carotene should be advised against taking supplementary vitamin A because beta carotene will fulfill normal vitamin A requirements. Patients should be cautioned that large quantities of green or yellow vegetables or their juices or extracts are not suitable substitutes for crystalline beta carotene because consumption of excessive quantities of these vegetables may cause adverse effects such as leukopenia or menstrual disorders. Patients should be warned that the protective effect of beta carotene is not total and that they may still develop considerable burning and edema after sufficient exposure to sunlight. Each patient must establish his own time limit of exposure.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3556
There are no adequate and controlled studies to date in humans. Beta carotene should be used during pregnancy only when the potential benefits justify the possible risks to the fetus. The effect of beta carotene on fertility in humans is not known.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3556
For more Drug Warnings (Complete) data for BETA-CAROTENE (11 total), please visit the HSDB record page.
Beta-carotene is FDA approved to be used as a nutrient supplement and to be even added in infant formula as a source of vitamin A. It is also approved to be used as a color additive for food products, drugs (with the label of "only as a color additive") and cosmetics. It is used commonly for the reduction of photosensitivity in patients with erythropoietic protoporphyria and other photosensitivity diseases.
Oral administration of beta-carotene increases the serum concentration of beta-carotene by 60% but it does not change the concentration found in the heart, liver or kidneys. In vitro studies in hepatocytes have shown that beta-carotene ameliorates oxidative stress, enhances antioxidant activity and decreases apoptosis. Other than the antioxidant activities, some other actions have been correlated to beta-carotene. It is thought to have detoxifying properties, as well as to help increase resistance to inflammation and infection and increase immune response and enhance RNA production.
Provitamins
Precursor forms of vitamins. (See all compounds classified as Provitamins.)
A - Alimentary tract and metabolism
A11 - Vitamins
A11C - Vitamin a and d, incl. combinations of the two
A11CA - Vitamin a, plain
A11CA02 - Betacarotene
D - Dermatologicals
D02 - Emollients and protectives
D02B - Protectives against uv-radiation
D02BB - Protectives against uv-radiation for systemic use
D02BB01 - Betacarotene
Absorption
After administration of beta-carotene, some of the administered dose is absorbed into the circulatory system unchanged and stored in the fat tissue. The coadministration of beta-carotene and a high-fat content diet is correlated to a better absorption of beta-carotene. The absorption is also dependent on the isomeric form of the molecule where the cis conformation seems to present a higher bioavailability. The absorption of beta-carotene is thought to be performed in 6-7 hours. The reported AUC of beta-carotene when administered orally from 0 to 440 hours after initial administration was reported to be 26.3 mcg.h/L. The maximal concentration of beta-carotene is attained in a dual pharmacokinetic profile after 6 hours and again after 32 hours with a concentration of 0.58 micromol/L.
Route of Elimination
The unabsorbed carotene is excreted in feces. It is also excreted in feces and urine as metabolites. The consumption of dietary fiber can increase the fecal excretion of fats and other fat-soluble compounds such as beta-carotene.
Volume of Distribution
No pharmacokinetic studies have been performed regarding the volume of distribution of beta-carotene.
Clearance
The clearance rate of beta-carotene administered orally is 0.68 nmol/L each hour.
Carotenoids are absorbed and transported via lymphatics to the liver. They circulate in association with lipoproteins, and are found in liver, adrenal, testes, and adipose tissue, and can be converted to vitamin A in numerous tissues, including the liver. Some beta carotene is absorbed as such and circulates in association with lipoproteins; it apparently partitions into body lipids and can be converted to vitamin A in numerous tissues, including the liver.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1781
Absorption of beta-carotene depends on the presence of dietary fat and bile in the intestinal tract.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
Unchanged beta-carotene is found in various tissues, primarily fat tissues, adrenal glands, and ovaries. Small concentrations are found in the liver.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
Only about one-third of beta-carotene or other carotenoids is absorbed by human beings. The absorption of carotenoids takes place in a relatively nonspecific fashion and depends upon the presence of bile and absorbable fat in the intestinal tract; it is greatly decreased by steatorrhea, chronic diarrhea, and very-low-fat diets.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1781
For more Absorption, Distribution and Excretion (Complete) data for BETA-CAROTENE (9 total), please visit the HSDB record page.
Beta-carotene is broken down in the mucosa of the small intestine and liver by beta-carotene dioxygenase to retinal which is a form of vitamin A. The function of this enzyme is vital as it decides if the beta-carotene is transformed to vitamin A or if it circulates in the plasma as beta-carotene. Less than a quarter of the ingested beta-carotene from root vegetables and about half of the beta-carotene from leafy green vegetables are converted to vitamin A.
A portion of the beta-carotene is converted to retinol in the wall of the small intestine, principally by its initial cleavage at the 15,15' double bond to form two molecules of retinal. Some of the retinal is further oxidized to retinoic acid; only one-half is reduced to retinol, which is then esterified and transported in the lymph. ...
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1781
Approximately 20 to 60% of beta-carotene is metabolized to retinaldehyde and then converted to retinol, primarily in the intestinal wall. A small amount of beta-carotene is converted to vitamin A in the liver. The proportion of beta-carotene converted to vitamin A diminishes inversely to the intake of beta-carotene, as long as the dosages are higher than one to two times the daily requirements. High doses of beta-carotene do not lead to abnormally high serum concentrations of vitamin A.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
Beta carotene may be converted to 2 molecules of retinal by cleavage at the 15-15' double bond in the center of the molecule. Most of the retinal is reduced to retinol which is then conjugated with glucuronic acid and excreted in urine and feces. Some retinal may be further oxidized to retinoic acid which can be decarboxylated and further metabolized, secreted into bile, and excreted in feces as the glucuronide.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3556
Two pathways have been suggested for the conversion of carotenoids to vitamin A in mammals, central cleavage and excentric cleavage. An enzyme, beta-carotenoid-15,15'-dioxygenase, has been partly purified from the intestines of several species and has been identified in several other organs and species. The enzyme, which converts beta-carotene into two molecules of retinal in good yield, requires molecular oxygen and is inhibited by sulfhydryl binding reagents and iron binding reagents. Most provitamin A carotenoids, including the beta-apo-carotenals, are cleaved to retinal by this enzyme. Its maximal activity in the rabbit is approximately 200 times that required to meet nutritional needs but is less than 50% of that expected to produce signs of vitamin A toxicity. Excentric cleavage unquestionably occurs in plants and some microorganisms and might occur in mammals. Thus far, however, carotenoid dioxygenase with excentric bond specificity has been identified in mammals, the yield of beta-apo-carotenals from beta-carotene in vivo and in vitro is very low, and beta-apo-carotenals are formed nonbiologically from beta-carotene.
PMID:2643691 Olson JA; J Nutr 119 (1): 105-8 (1989)
The carotenes are not converted to retinol very rapidly, so that overdoses of the carotenes do not cause vitamin A toxicity. /Carotenes/
Shoden & Griffin; Fundamentals of Clinical Nutrition: 77 (1980)
The apparent half-life of beta-carotene is of 6-11 days after initial administration.
Beta-carotene is an antioxidant that presents significant efficacy against the reactive oxygen species singlet oxygen. Beta-carotene acts as a scavenger of lipophilic radicals within the membranes of every cell compartments. It also presents an oxidative modification of LDL. The presence of long chains of conjugated double bonds is responsible for its antioxidative properties by allowing beta-carotene to chelate oxygen-free radicals and dissipate their energy. The chelation of free radicals inhibits the peroxidation of lipids. The effect of beta-carotene in the immune response is thought to be related to the direct effect on the thymus which increases the production of immune cells.
IN HEMATOPORPHYRIN PHOTOSENSITIZED MICE BETA-CAROTENE SHOWED PHOTOPROTECTION WAS DUE TO FREE RADICAL SCAVENGING OR SINGLET O QUENCHING BUT ALSO A POSSIBLE ROLE OF 400 NM LIGHT ABSORPTION, A PROPERTY OF BETA-CAROTENE.
PMID:839088 MOSHELL AN, BJORNSON L; J INVEST DERMATOL 68 (3): 157 (1977)
Beta carotene protects patients with erythropoietic protoporphyria against severe photosensitivity reactions (burning sensation, edema, erythema, pruritus, and/or cutaneous lesions). The drug has no effect on the basic biochemical abnormality of erythropoietic protoporphyria (eg, erythrocyte, plasma, and stool concentrations of protoporphyrins are not altered by the drug). The precise mechanism by which the drug exerts photoprotection has not been established. There is some evidence that photosensitizers may act through the formation of singlet excited oxygen and/or free radicals. Since in vitro studies indicate that beta carotene can quench free radicals and singlet excited oxygen, this may be the mechanism by which the drug acts. It is unlikely that beta carotene acts simply as a filter for the wavelengths of light that induce phototoxic effects.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3556
beta-Carotene inhibits UV-B carcinogenesis. beta-Carotene is an excellent quencher of singlet oxygen, and can quench free radicals. beta-Carotene has been shown to quench singlet oxygen/free radical reactions in the skin of porphyric mice, and has been found to quench excited species formed on irradiation of mouse skin by UV-B.
PMID:1881965 Black HS, Mathews-Roth MM; Photochem Photobiol 53 (5): 707-16 (1991)

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
69
PharmaCompass offers a list of Beta Carotene API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Beta Carotene manufacturer or Beta Carotene supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Beta Carotene manufacturer or Beta Carotene supplier.
PharmaCompass also assists you with knowing the Beta Carotene API Price utilized in the formulation of products. Beta Carotene API Price is not always fixed or binding as the Beta Carotene Price is obtained through a variety of data sources. The Beta Carotene Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Beta Carotene manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Beta Carotene, including repackagers and relabelers. The FDA regulates Beta Carotene manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Beta Carotene API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Beta Carotene manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Beta Carotene supplier is an individual or a company that provides Beta Carotene active pharmaceutical ingredient (API) or Beta Carotene finished formulations upon request. The Beta Carotene suppliers may include Beta Carotene API manufacturers, exporters, distributors and traders.
click here to find a list of Beta Carotene suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Beta Carotene DMF (Drug Master File) is a document detailing the whole manufacturing process of Beta Carotene active pharmaceutical ingredient (API) in detail. Different forms of Beta Carotene DMFs exist exist since differing nations have different regulations, such as Beta Carotene USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Beta Carotene DMF submitted to regulatory agencies in the US is known as a USDMF. Beta Carotene USDMF includes data on Beta Carotene's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Beta Carotene USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Beta Carotene suppliers with USDMF on PharmaCompass.
A Beta Carotene CEP of the European Pharmacopoeia monograph is often referred to as a Beta Carotene Certificate of Suitability (COS). The purpose of a Beta Carotene CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Beta Carotene EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Beta Carotene to their clients by showing that a Beta Carotene CEP has been issued for it. The manufacturer submits a Beta Carotene CEP (COS) as part of the market authorization procedure, and it takes on the role of a Beta Carotene CEP holder for the record. Additionally, the data presented in the Beta Carotene CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Beta Carotene DMF.
A Beta Carotene CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Beta Carotene CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Beta Carotene suppliers with CEP (COS) on PharmaCompass.
Beta Carotene Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Beta Carotene GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Beta Carotene GMP manufacturer or Beta Carotene GMP API supplier for your needs.
A Beta Carotene CoA (Certificate of Analysis) is a formal document that attests to Beta Carotene's compliance with Beta Carotene specifications and serves as a tool for batch-level quality control.
Beta Carotene CoA mostly includes findings from lab analyses of a specific batch. For each Beta Carotene CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Beta Carotene may be tested according to a variety of international standards, such as European Pharmacopoeia (Beta Carotene EP), Beta Carotene JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Beta Carotene USP).
