

Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
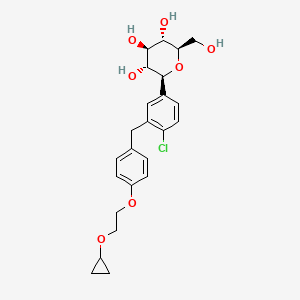

1. (2s,3r,4r,5s,6r)-2-(4-chloro-3-(4-(2-cyclopropoxyethoxy)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2h-pyran-3,4,5-triol
1. 1118567-05-7
2. Egt1442
3. Egt-1442
4. Thr-1442
5. Bexagliflozin [usan]
6. Egt0001442
7. Ey00jf42fv
8. (2s,3r,4r,5s,6r)-2-(4-chloro-3-(4-(2-cyclopropoxyethoxy)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2h-pyran-3,4,5-triol
9. Chembl1808388
10. Thr1442
11. Bexagliflozin (usan)
12. Egt-0001442
13. (2s,3r,4r,5s,6r)-2-(4-chloro-3-((4-(2-(cyclopropyloxy)ethoxy)phenyl)methyl)phenyl)-6-(hydroxymethyl)tetrahydro-2h-pyran-3,4,5-triol
14. (2s,3r,4r,5s,6r)-2-[4-chloro-3-[[4-(2-cyclopropyloxyethoxy)phenyl]methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol
15. D-glucitol, 1,5-anhydro-1-c-(4-chloro-3-((4-(2-(cyclopropyloxy)ethoxy)phenyl)methyl)phenyl)-, (1s)-
16. Unii-ey00jf42fv
17. Egt1442; Bexagliflozin
18. Bexagliflozin [inn]
19. Schembl302200
20. Bexagliflozin [who-dd]
21. Ex-a2332
22. Bdbm50349249
23. Zinc59047505
24. Bexagliflozin(thr1442egt1442)
25. Cs-5702
26. Db12236
27. (1s)-1,5-anhydro-1-c-[4-chloro-3-[[4-[2-(cyclopropyloxy)ethoxy]phenyl]methyl]phenyl]-d-glucitol
28. Ac-33643
29. As-55919
30. Hy-17604
31. C71393
32. D10865
33. J-500393
34. Q27277423
| Molecular Weight | 464.9 g/mol |
|---|---|
| Molecular Formula | C24H29ClO7 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 9 |
| Exact Mass | 464.1601810 g/mol |
| Monoisotopic Mass | 464.1601810 g/mol |
| Topological Polar Surface Area | 109 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 569 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of type II diabetes mellitus
Hypoglycemic Agents
Substances which lower blood glucose levels. (See all compounds classified as Hypoglycemic Agents.)
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 34601
Submission : 2020-03-30
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
Date of Issue : 2022-06-29
Valid Till : 2025-07-02
Written Confirmation Number : WC-0123
Address of the Firm : Sy. Nos. 7-70, 70/1 & 70/2, Digwal Village, Kohir Mandai, Sangareddy District, T...

Date of Issue : 2022-03-11
Valid Till : 2022-07-02
Written Confirmation Number : WC-0123A3
Address of the Firm : Sy. no 7-70,70/1,70/2, Digwal, Kohir,Sangareddy, Telangana state

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]NDC Package Code : 65085-0065
Start Marketing Date : 2018-09-26
End Marketing Date : 2024-12-31
Dosage Form (Strength) : POWDER (25kg/25kg)
Marketing Category : BULK INGREDIENT

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product ...
About the Company : Since its founding in 1962, MOEHS has produced Active Pharmaceutical Ingredients (APIs) for the international pharmaceutical industry. Thanks to a business history of more than 50 ...
 Jinan Tantu Chemicals offers customized R&D services & production of small molecule APIs & pharmaceutical intermediates.
Jinan Tantu Chemicals offers customized R&D services & production of small molecule APIs & pharmaceutical intermediates.
About the Company : Jinan Tantu Chemicals Co., Ltd. operates as a Contract Development and Manufacturing Organization (CDMO) that serves pharmaceutical companies worldwide. Our core services include c...
About the Company : Hope Chem is dedicated to manufacturing, marketing, and CMO service of Active Pharmaceutical Ingredients (APIs), Intermediates, and Specialty Chemicals since the year 2010. Current...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Brenzavvy (bexagliflozin) is an FDA-approved oral sodium-glucose cotransporter 2 (SGLT2) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
Lead Product(s): Bexagliflozin
Therapeutic Area: Endocrinology Brand Name: Brenzavvy
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable April 12, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bexagliflozin
Therapeutic Area : Endocrinology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Head-to-Head Study Finds TheracosBio's BRENZAVVY® (bexagliflozin) Non-Inferior to Dapagliflozin i...
Details : Brenzavvy (bexagliflozin) is an FDA-approved oral sodium-glucose cotransporter 2 (SGLT2) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes.
Brand Name : Brenzavvy
Molecule Type : Small molecule
Upfront Cash : Not Applicable
April 12, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the agreement, IPC gains distribution rights for Brenzavvy (bexagliflozin), an FDA-approved oral sodium-glucose cotransporter 2 (SGLT2) inhibitor, used for the treatment of adults with type 2 diabetes mellitus.
Lead Product(s): Bexagliflozin
Therapeutic Area: Endocrinology Brand Name: Brenzavvy
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Independent Pharmacy Cooperative
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Partnership February 01, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bexagliflozin
Therapeutic Area : Endocrinology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Independent Pharmacy Cooperative
Deal Size : Undisclosed
Deal Type : Partnership
Details : Under the agreement, IPC gains distribution rights for Brenzavvy (bexagliflozin), an FDA-approved oral sodium-glucose cotransporter 2 (SGLT2) inhibitor, used for the treatment of adults with type 2 diabetes mellitus.
Brand Name : Brenzavvy
Molecule Type : Small molecule
Upfront Cash : Undisclosed
February 01, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Under the collaboration, SmithRx will offer access to Brenzavvy (bexagliflozin), a sodium glucose transporter 2 (SGLT2) inhibitor and TheracosBio’s FDA-approved oral medication for adults with type 2 diabetes.
Lead Product(s): Bexagliflozin
Therapeutic Area: Endocrinology Brand Name: Brenzavvy
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: SmithRx
Deal Size: Undisclosed Upfront Cash: Undisclosed
Deal Type: Collaboration August 17, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bexagliflozin
Therapeutic Area : Endocrinology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : SmithRx
Deal Size : Undisclosed
Deal Type : Collaboration
TheracosBio and SmithRx Collaborate to Offer Newly Approved Diabetes Drug Brenzavvy™ (bexagliflo...
Details : Under the collaboration, SmithRx will offer access to Brenzavvy (bexagliflozin), a sodium glucose transporter 2 (SGLT2) inhibitor and TheracosBio’s FDA-approved oral medication for adults with type 2 diabetes.
Brand Name : Brenzavvy
Molecule Type : Small molecule
Upfront Cash : Undisclosed
August 17, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Brenzavvy (bexagliflozin), a SGLT2 inhibitor which helps in reducing renal reabsorption of filtered glucose and lowers the renal threshold for glucose, is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Lead Product(s): Bexagliflozin
Therapeutic Area: Endocrinology Brand Name: Brenzavvy
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable July 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bexagliflozin
Therapeutic Area : Endocrinology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Brenzavvy (bexagliflozin), a SGLT2 inhibitor which helps in reducing renal reabsorption of filtered glucose and lowers the renal threshold for glucose, is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diab...
Brand Name : Brenzavvy
Molecule Type : Small molecule
Upfront Cash : Not Applicable
July 13, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Brenzavvy™ (bexagliflozin) is a SGLT2 inhibitor thus, reducing renal reabsorption of filtered glucose and lowers the renal threshold for glucose, and thereby increases urinary glucose excretion.
Lead Product(s): Bexagliflozin
Therapeutic Area: Endocrinology Brand Name: Brenzavvy
Study Phase: ApprovedProduct Type: Small molecule
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable January 23, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bexagliflozin
Therapeutic Area : Endocrinology
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
TheracosBio Announces FDA Approval of Brenzavvy™ (bexagliflozin) for the Treatment of Adults wit...
Details : Brenzavvy™ (bexagliflozin) is a SGLT2 inhibitor thus, reducing renal reabsorption of filtered glucose and lowers the renal threshold for glucose, and thereby increases urinary glucose excretion.
Brand Name : Brenzavvy
Molecule Type : Small molecule
Upfront Cash : Not Applicable
January 23, 2023

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?
