
Synopsis
Synopsis
0
EU WC
0
KDMF
0
VMF
0
FDA Orange Book

0
Australia

DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
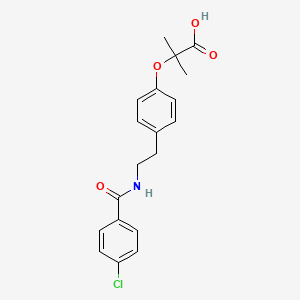

1. Azufibrat
2. Bfizal
3. Befibrat
4. Beza Lande
5. Beza Puren
6. Beza-lande
7. Beza-puren
8. Bezabeta
9. Bezacur
10. Bezafibrat Pb
11. Bezafisal
12. Bezalip
13. Bezamerck
14. Bm 15.075
15. Bm-15.075
16. Bm15.075
17. Cedur
18. Difaterol
19. Durabezur
20. Eulitop
21. Lipox
22. Reducterol
23. Regadrin B
24. Sklerofibrat
25. Solibay
1. 41859-67-0
2. Bezalip
3. Cedur
4. Bezafibrat
5. Difaterol
6. Befizal
7. Azufibrat
8. Sklerofibrat
9. Bezafibrato
10. Bezafibratum
11. Bezatol
12. Bezafibratum [inn-latin]
13. Bezafibrato [inn-spanish]
14. Bezatol Sr
15. Bm-15.075
16. Bezatol Sr (tn)
17. 2-[4-[2-[(4-chlorobenzoyl)amino]ethyl]phenoxy]-2-methylpropanoic Acid
18. Bm 15.075
19. 2-(p-(2-(p-chlorobenzamido)ethyl)phenoxy)-2-methylpropionic Acid
20. Bm-15075
21. Nsc-758174
22. 2-[4-[2-(4-chlorobenzamido)ethyl]phenoxy]-2-methylpropanoic Acid
23. Bm15075
24. Propanoic Acid, 2-(4-(2-((4-chlorobenzoyl)amino)ethyl)phenoxy)-2-methyl-
25. Mls000028533
26. Chembl264374
27. Durabezur
28. Reducterol
29. Bezabeta
30. Bezacur
31. Bezamerck
32. Eulitop
33. Solibay
34. Chebi:47612
35. Lipox
36. Bezafibrat Pb
37. Y9449q51xh
38. Beza-lande
39. Beza-puren
40. Regadrin B
41. 2-[4-[2-[(4-chlorobenzoyl)amino]ethyl]phenoxy]-2-methyl-propanoic Acid
42. Propanoic Acid, 2-[4-[2-[(4-chlorobenzoyl)amino]ethyl]phenoxy]-2-methyl-
43. Ncgc00016850-01
44. Smr000058298
45. Bezafibrate 100 Microg/ml In Acetonitrile
46. Bezafibrato [spanish]
47. 2-(4-(2-(4-chlorobenzamido)ethyl)phenoxy)-2-methylpropanoic Acid
48. Bezalip Retard
49. Cas-41859-67-0
50. Dsstox_cid_9869
51. Dsstox_rid_78826
52. Dsstox_gsid_29869
53. 2-{4-[2-(4-chlorobenzamido)ethyl]phenoxy}-2-methylpropanoic Acid
54. Lo 44
55. 2-(4-{2-[(4-chlorobenzoyl)amino]ethyl}phenoxy)-2-methylpropanoic Acid
56. 2-(4-{2-[(4-chlorophenyl)formamido]ethyl}phenoxy)-2-methylpropanoic Acid
57. 2-[p-[2-p-chlorobenzamido)ethyl]phenoxy]-2-methylpropionic Acid
58. Bf-759
59. Ccris 9085
60. Bm 15075
61. Sr-01000000106
62. Einecs 255-567-9
63. Brn 4267656
64. Unii-y9449q51xh
65. Bezalip Sr
66. Bezafibrate,(s)
67. Bezafibrate [usan:inn:ban:jan]
68. Pem
69. Prestwick_724
70. Mfcd00078970
71. Benafibrate
72. Spectrum_001443
73. Opera_id_376
74. Bezafibrate [mi]
75. Prestwick0_000378
76. Prestwick1_000378
77. Prestwick2_000378
78. Prestwick3_000378
79. Spectrum2_000922
80. Spectrum3_001500
81. Spectrum4_000325
82. Spectrum5_001079
83. Spectrum5_001967
84. Bezafibrate [inn]
85. Bezafibrate [jan]
86. Bezafibrate [usan]
87. Bezafibrate [mart.]
88. Schembl16299
89. Bezafibrate [who-dd]
90. Bezafibrate-d6(dimethyl-d6)
91. Bspbio_000535
92. Bspbio_001314
93. Bspbio_003119
94. Kbiogr_000034
95. Kbiogr_000669
96. Kbioss_000034
97. Kbioss_001923
98. Mls001148205
99. Bezafibrate, >=98%, Solid
100. Divk1c_000092
101. Spectrum1502046
102. Spbio_000824
103. Spbio_002456
104. Bpbio1_000589
105. Gtpl2668
106. Dtxsid3029869
107. Bdbm28701
108. Bezafibrate (jp17/usan/inn)
109. Hms500e14
110. Kbio1_000092
111. Kbio2_000034
112. Kbio2_001923
113. Kbio2_002602
114. Kbio2_004491
115. Kbio2_005170
116. Kbio2_007059
117. Kbio3_000067
118. Kbio3_000068
119. Kbio3_002619
120. 2-[4-[2-(4-chlorobenzamido)ethyl]phenoxy]isobutyric Acid
121. Ninds_000092
122. Bezafibrate [ep Monograph]
123. Bio2_000034
124. Bio2_000514
125. Hms1361b16
126. Hms1569k17
127. Hms1791b16
128. Hms1921h16
129. Hms1989b16
130. Hms2089f04
131. Hms2092b12
132. Hms2096k17
133. Hms2233e22
134. Hms3261d21
135. Hms3369b13
136. Hms3402b16
137. Hms3650k22
138. Hms3652m22
139. Hms3713k17
140. Pharmakon1600-01502046
141. Bcp03700
142. Hy-b0637
143. Zinc3956919
144. Tox21_110645
145. Tox21_301845
146. Tox21_500500
147. Ccg-39683
148. Nsc758174
149. S4159
150. Akos005107743
151. Propionic Acid, 2-(4-(2-((4-chlorobenzoyl)amino)ethyl)phenoxy)-2-methyl-
152. Tox21_110645_1
153. Ab03023
154. Ac-6817
155. Bcp9000398
156. Db01393
157. Hs-0040
158. Lp00500
159. Nsc 758174
160. Sb17361
161. Sdccgsbi-0051715.p003
162. Idi1_000092
163. Idi1_033784
164. Ncgc00016850-02
165. Ncgc00016850-03
166. Ncgc00016850-04
167. Ncgc00016850-05
168. Ncgc00016850-06
169. Ncgc00016850-07
170. Ncgc00016850-08
171. Ncgc00016850-09
172. Ncgc00016850-10
173. Ncgc00016850-11
174. Ncgc00016850-12
175. Ncgc00016850-15
176. Ncgc00016850-25
177. Ncgc00023317-03
178. Ncgc00023317-04
179. Ncgc00023317-05
180. Ncgc00023317-06
181. Ncgc00023317-07
182. Ncgc00023317-08
183. Ncgc00255376-01
184. Ncgc00261185-01
185. Bb166159
186. Bezafibrate, Analytical Reference Material
187. Bcp0726000153
188. Sbi-0051715.p002
189. 2-(4-(2-(4-chlorobenzamido)ethyl)phenoxy)
190. Ab00052265
191. B3346
192. Ft-0622617
193. Sw196871-4
194. D01366
195. D70191
196. Ab00052265-15
197. Ab00052265_16
198. Ab00052265_17
199. 859b670
200. Q577387
201. Sr-01000000106-3
202. Sr-01000000106-4
203. Sr-01000000106-5
204. W-106291
205. Brd-k46018455-001-06-0
206. Brd-k46018455-001-17-7
207. Sr-01000000106-10
208. Bezafibrate, European Pharmacopoeia (ep) Reference Standard
209. 2-(4-(2-parachlorobenzamidoethyl)phenoxy)-2-methylpropionic Acid
210. 2-[4-[2-(4-chlorobezamide)ethyl]phenoxy]-2-methylpropanoic Acid
211. 2-[4-(2-{[(4-chlorophenyl)carbonyl]amino}ethyl)phenoxy]-2-methylpropanoic Acid
212. Bf; 2-[4-[2-[(4-chlorobenzoyl)amino]ethyl]phenoxy]-2-methyl Propanoic Acid
| Molecular Weight | 361.8 g/mol |
|---|---|
| Molecular Formula | C19H20ClNO4 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 361.1080858 g/mol |
| Monoisotopic Mass | 361.1080858 g/mol |
| Topological Polar Surface Area | 75.6 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 452 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of primary hyperlipidaemia types IIa, IIb, III, IV and V (Fredrickson classification) corresponding to groups I, II and III of the European Atherosclerosis Society guidelines - when diet alone or improvements in lifestyle such as increased exercise or weight reduction do not lead to an adequate response. Also for the treatment of secondary hyperlipidaemias, e.g. severe hypertriglyceridemias, when sufficient improvement does not occur after correction of the underlying disorder (e.g. diabetes mellitus).
Bezafibrate is an antilipemic agent that lowers cholesterol and triglycerides. It decreases low density lipoproteins and increases high density lipoproteins. Bezafibrate lowers elevated blood lipids (triglycerides and cholesterol). Elevated VLDL and LDL are reduced by treatment with bezafibrate, whilst HDL-levels are increased. The activity of triglyceride lipases (lipoprotein lipase and hepatic lipoproteinlipase) involved in the catabolism of triglyceride-rich lipoproteins is increased by bezafibrate. In the course of the intensified degradation of triglyceride-rich lipoproteins (chylomicrons, VLDL) precursors for the formation of HDL are formed which explains an increase in HDL. Furthermore, cholesterol biosynthesis is reduced by bezafibrate, which is accompanied by a stimulation of the LDL-receptor-mediated lipoprotein catabolism. Elevated fibrinogen appears to be an important risk-factor, alongside the lipids, smoking and hypertension, in the development of atheroma. Fibrinogen plays an important role in viscosity, and therefore blood flow, and also appears to play an important role in thrombus development and lysability. Bezafibrate exerts an effect on thrombogenic factors. A significant decrease in elevated plasma fibrinogen levels can be achieved. This may lead, amongst other things, to a reduction in both blood and plasma viscosity. Inhibition of platelet aggregation has also been observed. A reduction in blood glucose concentration due to an increase in glucose tolerance has been reported in diabetic patients. In the same patients, the concentration of fasting and postprandial free fatty acids was reduced by bezafibrate.
Hypolipidemic Agents
Substances that lower the levels of certain LIPIDS in the BLOOD. They are used to treat HYPERLIPIDEMIAS. (See all compounds classified as Hypolipidemic Agents.)
C10AB02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C10AB02
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
C10AB02
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
C - Cardiovascular system
C10 - Lipid modifying agents
C10A - Lipid modifying agents, plain
C10AB - Fibrates
C10AB02 - Bezafibrate
Absorption
Bezafibrate is almost completely absorbed after oral administration. The relative bioavailability of bezafibrate retard compared to the standard form is about 70%.
Hepatic.
1-2 hours
It is generally accepted that bezafibrate is likely an agonist of PPAR-alpha. However, certain other investigations have also suggested that the substance might also elicit some effects on PPAR-gamma and PPAR-delta too.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
92
PharmaCompass offers a list of Bezafibrate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Bezafibrate manufacturer or Bezafibrate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Bezafibrate manufacturer or Bezafibrate supplier.
PharmaCompass also assists you with knowing the Bezafibrate API Price utilized in the formulation of products. Bezafibrate API Price is not always fixed or binding as the Bezafibrate Price is obtained through a variety of data sources. The Bezafibrate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Bezafibrate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Bezafibrate, including repackagers and relabelers. The FDA regulates Bezafibrate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Bezafibrate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Bezafibrate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Bezafibrate supplier is an individual or a company that provides Bezafibrate active pharmaceutical ingredient (API) or Bezafibrate finished formulations upon request. The Bezafibrate suppliers may include Bezafibrate API manufacturers, exporters, distributors and traders.
click here to find a list of Bezafibrate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Bezafibrate DMF (Drug Master File) is a document detailing the whole manufacturing process of Bezafibrate active pharmaceutical ingredient (API) in detail. Different forms of Bezafibrate DMFs exist exist since differing nations have different regulations, such as Bezafibrate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Bezafibrate DMF submitted to regulatory agencies in the US is known as a USDMF. Bezafibrate USDMF includes data on Bezafibrate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Bezafibrate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Bezafibrate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Bezafibrate Drug Master File in Japan (Bezafibrate JDMF) empowers Bezafibrate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Bezafibrate JDMF during the approval evaluation for pharmaceutical products. At the time of Bezafibrate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Bezafibrate suppliers with JDMF on PharmaCompass.
A Bezafibrate CEP of the European Pharmacopoeia monograph is often referred to as a Bezafibrate Certificate of Suitability (COS). The purpose of a Bezafibrate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Bezafibrate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Bezafibrate to their clients by showing that a Bezafibrate CEP has been issued for it. The manufacturer submits a Bezafibrate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Bezafibrate CEP holder for the record. Additionally, the data presented in the Bezafibrate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Bezafibrate DMF.
A Bezafibrate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Bezafibrate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Bezafibrate suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Bezafibrate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Bezafibrate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Bezafibrate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Bezafibrate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Bezafibrate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Bezafibrate suppliers with NDC on PharmaCompass.
Bezafibrate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Bezafibrate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Bezafibrate GMP manufacturer or Bezafibrate GMP API supplier for your needs.
A Bezafibrate CoA (Certificate of Analysis) is a formal document that attests to Bezafibrate's compliance with Bezafibrate specifications and serves as a tool for batch-level quality control.
Bezafibrate CoA mostly includes findings from lab analyses of a specific batch. For each Bezafibrate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Bezafibrate may be tested according to a variety of international standards, such as European Pharmacopoeia (Bezafibrate EP), Bezafibrate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Bezafibrate USP).
