
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
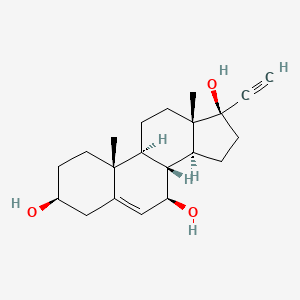

1. 17-ethynyl-5-androstene-3, 7, 17-triol
2. 17-ethynyl-5-androstene-3,7,17-triol
3. He-3286
4. He3286
1. He3286
2. 1001100-69-1
3. He 3286
4. Bezisterim
5. He-3286
6. Unii-ph8858757i
7. Pregn-5-en-20-yne-3,7,17-triol, (3beta,7beta,17alpha)-
8. Ph8858757i
9. 3beta,7beta,17beta-trihydroxy-17alpha-ethynylandrost-5-ene
10. (3s,7r,8r,9s,10r,13s,14s,17r)-17-ethynyl-10,13-dimethyl-1,2,3,4,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthrene-3,7,17-triol
11. Bezisterim [inn]
12. 17-ethynyl-5-androstene-3, 7, 17-triol
13. Schembl512549
14. Chembl4297284
15. Dtxsid501267252
16. Akos040749677
17. Db05212
18. Hy-108039
19. Cs-0027210
20. Ns00072554
21. G13918
22. 17alpha-pregn-5-en-20-yne-3beta,7beta,17-triol
23. Q27286562
24. 17.alpha.-ethynyl-5-androstene-3.beta.,7.beta.,17.beta.-triol
25. Pregn-5-en-20-yne-3,7,17-triol, (3.beta.,7.beta.,17.alpha.)-
| Molecular Weight | 330.5 g/mol |
|---|---|
| Molecular Formula | C21H30O3 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | g/mol |
| Monoisotopic Mass | g/mol |
| Topological Polar Surface Area | 60.7 |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 630 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of rheumatoid arthritis and type 2 diabetes.
Absorption
Up to 25% oral bioavailability in mice.
Potential mechanisms of action for HE3286 include regulation of NF-kB and increasing the production of regulatory T cells (Treg cells). NF-kB is a well-known transcription factor that controls the production of inflammatory cytokines such as TNF-a and interferon-g. Treg cells are referred to in the scientific literature as the peacekeepers of the body. Their role is to keep the immune system from attacking the body itself. Recent studies of Treg cells indicate that they may play a broader role than simply preventing autoimmune conditions. Manipulation of these cells may offer new treatments for conditions ranging from diabetes and organ rejection to cancer and infectious diseases. In type II diabetes, moderate inhibition of NF-kB improves glucose tolerance. [Press Release - Hollis-Eden Pharmaceuticals]
ABOUT THIS PAGE
