
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
API
0
FDF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Weekly News Recap #Phispers
US Medicaid
NA
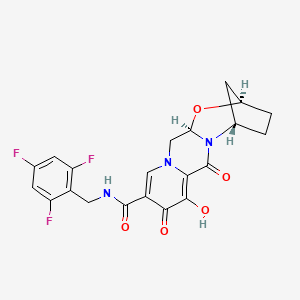

1. Bictegravir Sodium
2. Gs-9883
3. Gs-9883 Sodium
4. Gs-9883-01
5. Gs-9883-01 Sodium
1. 1611493-60-7
2. Gs-9883
3. Bictegravir [inn]
4. Gs-9883-01
5. (2r,5s,13ar)-8-hydroxy-7,9-dioxo-n-(2,4,6-trifluorobenzyl)-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxamide
6. 8gb79loj07
7. (1s,11r,13r)-5-hydroxy-3,6-dioxo-n-[(2,4,6-trifluorophenyl)methyl]-12-oxa-2,9-diazatetracyclo[11.2.1.02,11.04,9]hexadeca-4,7-diene-7-carboxamide
8. 2,5-methanopyrido(1',2':4,5)pyrazino(2,1-b)(1,3)oxazepine-10-carboxamide, 2,3,4,5,7,9,13,13a-octahydro-8-hydroxy-7,9-dioxo-n-((2,4,6-trifluorophenyl)methyl)-, (2r,5s,13ar)-
9. Bictegravir [usan:inn]
10. Bictegravirum
11. Unii-8gb79loj07
12. Gs 9883
13. Bictegravir [mi]
14. Bictegravir (usan/inn)
15. Bictegravir [usan]
16. Bictegravir [who-dd]
17. Chembl3989866
18. Schembl15914278
19. Gtpl11575
20. Chebi:172943
21. Bdbm330048
22. Dtxsid701027937
23. Amy12383
24. Bcp25703
25. Ex-a3161
26. Gs9883
27. S5911
28. Db11799
29. Dt-0020
30. Ac-30658
31. Hy-17605
32. Cs-0014685
33. D10909
34. N16998
35. Us9663528, 42
36. A902376
37. Q27270406
38. Gs-9883; Gs 9883; Gs9883; Gs-9883-01
39. (1s,11r,13r)-5-hydroxy-3,6-dioxo-n-[(2,4,6-trifluorophenyl)methyl]-12-oxa-2,9-diazatetracyclo[11.2.1.0(2),(1)(1).0?,?]hexadeca-4,7-diene-7-carboxamide
40. (2r,5s,13ar)-8-hydroxy-7,9-dioxo-n-(2,4,6-trifluorobenzyl)-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2, 1-b][1,3]oxazepine-10-carboxamide
| Molecular Weight | 449.4 g/mol |
|---|---|
| Molecular Formula | C21H18F3N3O5 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 3 |
| Exact Mass | 449.11985517 g/mol |
| Monoisotopic Mass | 449.11985517 g/mol |
| Topological Polar Surface Area | 99.2 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 912 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Bictegravir is indicated in the management of HIV-1 infection in patients not previously treated with antiretroviral therapy. Additionally, Bictegravir is indicated in the management of HIV-1 infection in patients who are virologically suppressed (HIV-1 RNA <50 c/mL) on a regular antiretroviral regimen for a minimum of three months without a history of failure in treatment and no known factors associated with the resistance to the individual components of the medication. It is used in combination with tenofovir and emtricitabine.
FDA Label
Bictegravir is an HIV-1 integrase strand transfer inhibitor (INSTI). Bictegravir (BIC) inhibits HIV-1 virus replication into the human genome. It can be taken once daily without additional dosing. Bictegravir (BIC) inhibits strand transfer of viral DNA into the host genome and thereby prevents HIV-1 replication.
Absorption
Bictegravir is rapidly absorbed within the body. Tmax= 2.0-4.0h [FDA LABEL, 1219]
Route of Elimination
BIC is mainly eliminated through UGT1A1 glucuronidation and CYP3A4 oxidation, equally. About 1% of the bictegravir dose is excreted in the urine, unchanged [1218,1219].
Volume of Distribution
0.2 L/Kg in humans
Clearance
Bictegravir is mainly cleared by the kidneys. Those with renal clearance <30 should not take bictegravir [FDA LABEL]
In a 10-day dose-ranging study, monotherapy (5 mg to 100 mg) once daily in adults who were not previously treated with bictegravir, the median half-life of BIC ranged from 15.9 h - 20.9 h [L1219. Bictegravir is metabolized in the liver and kidneys. CYP3A4 and UGT1A are the primary enzymes involved in the metabolism of bictegravir. Administration of bictegravir is not advised in patients with renal creatinine clearance of <30 mL/min and patients with hepatic disease [FDA LABEL].
17. 3h [FDA LABEL, L1219]
This single dose medication inhibits the strand transfer of viral DNA into the human genome, preventing HIV-1 virus replication and propagation. In vitro, bictegravir has shown powerful antiviral activity against HIV-2 and various subtypes of HIV-1. It has shown synergistic effects when combined with other ARVs, including tenofovir alafenamide (TAF), emtricitabine (FTC), and darunavir (DRV) [FDA LABEL]. The three components of the first USA approved medication ( trade name: Biktarvy ) are as follows: Bictegravir: integrase strand transfer inhibitor; INSTI), an HIV-1 encoded enzyme necessary for viral replication. Inhibition of the integrase enzyme prevents the integration of HIV-1 into host DNA, blocking the conversion of the HIV-1 provirus and progression of the virus [FDA LABEL]. Emtricitabine: FTC, is phosphorylated by cellular enzymes to form emtricitabine 5'-triphosphate. Emtricitabine is phosphorylated to form emtricitabine 5'-triphosphate intracellularly. This metabolite inhibits the activity of human immunodeficiency virus (HIV) reverse transcriptase by competing with the substrate deoxycytidine 5'-triphosphate and by incorporating itself into viral DNA preventing DNA chain elongation [FDA LABEL]. Tenofovir Alafenamide: TAF is a phosphonamidate prodrug of tenofovir (2-deoxyadenosine monophosphate analog). Plasma exposure to TAF leads to leakage into cells and then TAF is intracellularly converted to tenofovir by hydrolysis by cathepsin. Tenofovir is subsequently phosphorylated by cellular kinases to the metabolite tenofovir diphosphate, which is the active form of the drug. Tenofovir diphosphate inhibits HIV-1 replication by incorporating into viral DNA by the HIV reverse transcriptase, resulting in DNA chain-termination. Tenofovir diphosphate also weakly inhibits mammalian DNA polymerases [FDA LABEL].
CAS Number : 214759-21-4
End Use API : Bictegravir
About The Company : Shanghai fine pharma founded in 2014, is a professional enterprise with chemical reagents and fine chemical products development, development and trade. The com...

(1R,3S)-3-Aminocyclopentanol hydrochloride
CAS Number : 1279032-31-3
End Use API : Bictegravir
About The Company : Shanghai fine pharma founded in 2014, is a professional enterprise with chemical reagents and fine chemical products development, development and trade. The com...

CAS Number : 1110772-05-8
End Use API : Bictegravir
About The Company : Shanghai fine pharma founded in 2014, is a professional enterprise with chemical reagents and fine chemical products development, development and trade. The com...

3-Aminocyclopentanol hydrochloride
CAS Number : 1184919-69-4
End Use API : Bictegravir
About The Company : Shanghai fine pharma founded in 2014, is a professional enterprise with chemical reagents and fine chemical products development, development and trade. The com...

(1R,3S)-3-aminocyclopentan-1-ol hydrochloride
CAS Number : 1279032-31-3
End Use API : Bictegravir
About The Company : Haorui, established in 1997, is a manufacturer and supplier of APIs, intermediates, veterinary and food additives. Meanwhile, it is assisting global pharmaceuti...

CAS Number : 1110772-05-8
End Use API : Bictegravir
About The Company : Shenzhen HwaGen Pharmaceutical Co., Ltd. (HwaGen Pharma) was founded in December of 2015 under the leadership of a “National 1000 Talents Program” expert. B...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?