
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
VMF
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
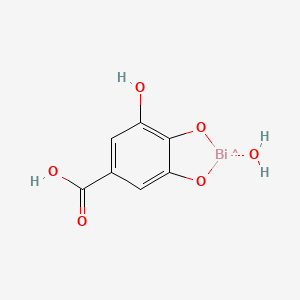

1. Bismuth Subgallate Hydrate
2. Dermatol Ointment
1. 99-26-3
2. Bismuthsubgallate
3. Wismutgallathydroxid
4. Gallic Acid Bismuth Basic Salt
5. Bismuth Gallate
6. Dermatol Puder
7. 2,7-dihydroxy-1,3,2-benzodioxabismole-5-carboxylic Acid
8. Basic Bismuth 3,4,5-trihydroxybenzoate
9. Ncgc00166280-01
10. Bismuth Subgallas
11. Bismutum Subgallicum
12. Wismutgallat, Basisches
13. Caswell No. 098b
14. 2,7-dihydroxybenzo[d][1,3,2]dioxabismole-5-carboxylic Acid
15. Dermatol
16. Bismuth(iii) Gallate Basic Hydrate
17. Einecs 202-742-2
18. Unii-yiw503mi7v
19. Epa Pesticide Chemical Code 098601
20. Bismuth-subgallate
21. Dermatol (tn)
22. Bismuth Subgallate [usan:usp:jan]
23. Basisches Wismutgallat
24. 1,3,2-benzodioxabismole-5-carboxylic Acid, 2,7-dihydroxy-
25. Bismuth 3,4,5-trihydroxybenzoate, Basic
26. Dihydroxy(3,4,5-trihydroxybenzoato-o')bismuth
27. Schembl7892
28. Dsstox_cid_26588
29. Dsstox_rid_81746
30. Dsstox_gsid_46588
31. Bismuth, Dihydroxy(3,4,5-trihydroxybenzoato-o')-
32. Dtxsid3046588
33. Bismuth Subgallate (jp17/usp)
34. Chebi:31292
35. Bismuth(iii) Gallate, Basic
36. Hy-b1560
37. Tox21_112388
38. Mfcd00044980
39. Db13909
40. Cas-99-26-3
41. Ncgc00166280-02
42. Cs-0013442
43. D01398
44. E75771
| Molecular Weight | 395.10 g/mol |
|---|---|
| Molecular Formula | C7H6BiO6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 1 |
| Exact Mass | 394.99684 g/mol |
| Monoisotopic Mass | 394.99684 g/mol |
| Topological Polar Surface Area | 77 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 222 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
The most common medical purpose for which bismuth subgallate is currently and formally indicated for is the use as a non-prescription internal deodorant product for the purpose of deodorizing flatulence and stools. Additionally, there are also various non-prescription (over the counter) bismuth subgallate based wound healing products as well as ongoing studies into whether or not the substance can be utilized as a legitimate hemostatic agent - usually for soft tissue surgery in otorhinolaryngology and/or dermatologic settings. Moreover, in the past bismuth subgallate may have seen some use as a treatment for Helicobacter pylori infection. In contrast, contemporary first-line therapies generally involve proton pump inhibitor and antibiotic combination therapies that generally achieve high rates of pathogen eradication, ease of administration, and patient compliance.
Bismuth subgallate is a heavy metal salt that is relatively insoluble and poorly absorbed. As a result, systemic absorption is not necessary or possibly even desired when the agent is administered orally or onto specific otorhinolaryngology and/or dermatologic wound sites where it can execute its pharmacologic action directly within the gastrointestinal lumen to deodorize flatulence and stools or potentially elicit a hemostatic effect on wounds. Additionally, like other bismuth agents, one of the most common side effects associated with bismuth subgallate is its propensity to cause a black discoloration of the tongue and stools when the agent combines with trace amounts of sulfur in the saliva and/or gastrointestinal tract. This discoloration is temporary and harmless, gradually dissipating over a number of days and eventually disappearing after the discontinuation of the bismuth agent.
Anti-Infective Agents
Substances that prevent infectious agents or organisms from spreading or kill infectious agents in order to prevent the spread of infection. (See all compounds classified as Anti-Infective Agents.)
Hemostatics
Agents acting to arrest the flow of blood. Absorbable hemostatics arrest bleeding either by the formation of an artificial clot or by providing a mechanical matrix that facilitates clotting when applied directly to the bleeding surface. These agents function more at the capillary level and are not effective at stemming arterial or venous bleeding under any significant intravascular pressure. (See all compounds classified as Hemostatics.)
Absorption
Bismuth subgallate is only slightly, if at all, absorbed after oral ingestion. The general human oral bioavailability of bismuth subgallate has been reported as low as 0.04%. Any absorption that does occur is likely to happen from the upper small intestine. The gastrointestinal absorption of bismuth from bismuth compounds demonstrates a large interindividual variation. Factors affecting the absorption involve the formulation of the bismuth subgallate compound as well as the dietary factors of the individuals themselves. Nevertheless, absorption can be enhanced with the concomitant intake of citrate and sulfhydryl-group-containing compounds. Conversely, the simultaneous administration of antacids or a diet that is high in thiol content can lower absorption of bismuth subgallate.
Route of Elimination
Ingested bismuth is primarily eliminated unabsorbed by way of the faeces. Any absorbed bismuth is eliminated from the body by both the urinary and faecal (including bile) routes. Excretion of absorbed bismuth in the urine is rapid, with most of the metal excreted within 24 hours. About 10% of the absorbed bismuth is detected in faeces, presumably owing to biliary secretion.
Volume of Distribution
In general, oral administration is one of the most common routes of administration for non-prescription bismuth subgallate products and gastrointestinal and systemic absorption is usually very low.
Clearance
On average, the blood clearance of the bismuth component of a bismuth salt like bismuth subgallate is within the range of 50 to 95 ml/min.
No specific metabolism of bismuth is known. In the kidney it induces the de novo synthesis of a bismuth-metal-binding protein, which is a kind of methallothionein.
The bismuth component of bismuth subgallate is known to have a terminal half-life of 21-72 days.
Bismuth salts exert their action largely in the upper gastrointestinal tract by way of local activity from luminal bismuth in the stomach and duodenum. In terms of bismuth subgallate's ability to deodorize flatulence and stools as an internal deodorant - although not fully elucidated - it is believed that when the substance is administered orally, its relative insolubility and poor absorption allows it to remain within the gastrointestinal lumen and inhibit colonic bacteria from acting on fermentable food residues in the GI tract. Moreover, when bismuth subgallate is taken orally, various salts like bismuth citrate, bismuth oxychloride, and others are formed. These salts are then taken up into surrounding gastric mucus as well as bound to protein within the base of any ulcers that may be present after coming into contact with gastric juice. Additionally, bismuth compounds like bismuth subgallate are also believed to have the capacity to trigger the secretion of prostaglandins, epithelial growth factor (EGF), and mucosal bicarbonate as a means to inhibit the action of pepsin in gastric juice. These actions subsequently protect gastric mucous from peptic luminal degradation as well as enhance the properties of mucous to assist in the healing of both duodenal and gastric ulcers. In this way, bismuth subgallate works to absorb extra water and/or toxins in the large intestine, allowing it to form a protective coat on the intestinal mucosa and over ulcers that may or may not be associated with infections like those of Helicobacter pylori. Furthermore, studies have shown that bismuth compounds like bismuth subgallate are capable of demonstrating antimicrobial effects against various gastrointestinal tract pathogens like E. coli, Salmonella, Shigella, Vibrio cholera, Campylobacter jejuni, H. pylori, and some enteric viruses like Rotaviruses. Although the exact mechanism(s) of action by which bismuth compounds are able to elicit such antimicrobial effects remains unclear, a number of experimental observations suggest that bismuth has been able to complex with the bacterial wall and periplasmic membrane; inhibit bacterial enzymes like urease, catalase, and lipase; inhibit bacterial protein and ATP synthesis; and also inhibit or decrease the adherence of bacteria like H. pylori to epithelial cells. In essence, ultrastructural studies have shown evidence of the binding of bismuth complexes to the bacterial wall and periplasmic space between the inner and outer bacterial membrane of H.pylori with subsequent ballooning and disintegration of the pathogen. To various extents, these antimicrobial actions may also illustrate how bismuth subgallate is capable of neutralizing colonic bacteria from acting on fermentable foods as well. Numerous studies have and continue to study the possible hemostatic action that bismuth subgallate may have. As the bismuth salt of gallic acid, bismuth subgallate's chemical structure shares similarities to ellagic acid, another gallic acid derivative. Ellagic acid itself is a clot-promoting agent that initiates thrombin formation by way of the intrinsic pathway via an action on Hageman factor (clotting factor XII). It is believed that bismuth subgallate's ability to activate factor XII is associated with the chemical's negatively charged moieties - whose contact with factor XII would theoretically initiate the intrinsic pathway to blood clotting. Other studies have also suggested that bismuth subgallate is capable of inducing macrophages to secrete growth factors to facilitate wound healing, decreasing lesion area, enhancing granulation tissue formation and re-epithelialization, the initiation of the proliferation of collagen via the activation of fibroblasts, the accelerated re-establishment of blood vessels, and also the restriction of nitric oxide formation. Given such studies regarding bismuth subgallate's potential hemostatic abilities, there has been and continues to be interest in indicating the agent for use in otolaryngology as in tonsillectomies or adenotonsillectomies to achieve reduced bleeding and surgery times; topical treatment in various open wound surgeries to facilitate faster and earlier clotting between tissues; ileostomy; dental surgeries; epistaxis management; among others. Nevertheless, study results are conflicting; where there may be experimental results suggesting some improvements in reduced operation time and operative blood loss when bismuth subgallate is used as a hemostatic agent in tonsillectomies there are also study results that observed bismuth subgallate having a negative influence on the healing processes of wounds inflicted in animal models, in which the use of the agent actually delayed the rate of new vessel formation and optimal wound healing. Finally, bismuth subgallate also demonstrates a strong astringent ability - an action that can facilitate both its deodorant and hemostatic effects and assists in its indication as an active ingredient in a number of non-prescription products for hemorrhoid suppositories or topical applications, diarrhea, etc.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
19
PharmaCompass offers a list of Bismuth Subgallate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Bismuth Subgallate manufacturer or Bismuth Subgallate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Bismuth Subgallate manufacturer or Bismuth Subgallate supplier.
PharmaCompass also assists you with knowing the Bismuth Subgallate API Price utilized in the formulation of products. Bismuth Subgallate API Price is not always fixed or binding as the Bismuth Subgallate Price is obtained through a variety of data sources. The Bismuth Subgallate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Bismuth Subgallate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Bismuth Subgallate, including repackagers and relabelers. The FDA regulates Bismuth Subgallate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Bismuth Subgallate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Bismuth Subgallate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Bismuth Subgallate supplier is an individual or a company that provides Bismuth Subgallate active pharmaceutical ingredient (API) or Bismuth Subgallate finished formulations upon request. The Bismuth Subgallate suppliers may include Bismuth Subgallate API manufacturers, exporters, distributors and traders.
click here to find a list of Bismuth Subgallate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Bismuth Subgallate DMF (Drug Master File) is a document detailing the whole manufacturing process of Bismuth Subgallate active pharmaceutical ingredient (API) in detail. Different forms of Bismuth Subgallate DMFs exist exist since differing nations have different regulations, such as Bismuth Subgallate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Bismuth Subgallate DMF submitted to regulatory agencies in the US is known as a USDMF. Bismuth Subgallate USDMF includes data on Bismuth Subgallate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Bismuth Subgallate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Bismuth Subgallate suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Bismuth Subgallate Drug Master File in Japan (Bismuth Subgallate JDMF) empowers Bismuth Subgallate API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Bismuth Subgallate JDMF during the approval evaluation for pharmaceutical products. At the time of Bismuth Subgallate JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Bismuth Subgallate suppliers with JDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Bismuth Subgallate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Bismuth Subgallate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Bismuth Subgallate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Bismuth Subgallate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Bismuth Subgallate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Bismuth Subgallate suppliers with NDC on PharmaCompass.
Bismuth Subgallate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Bismuth Subgallate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Bismuth Subgallate GMP manufacturer or Bismuth Subgallate GMP API supplier for your needs.
A Bismuth Subgallate CoA (Certificate of Analysis) is a formal document that attests to Bismuth Subgallate's compliance with Bismuth Subgallate specifications and serves as a tool for batch-level quality control.
Bismuth Subgallate CoA mostly includes findings from lab analyses of a specific batch. For each Bismuth Subgallate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Bismuth Subgallate may be tested according to a variety of international standards, such as European Pharmacopoeia (Bismuth Subgallate EP), Bismuth Subgallate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Bismuth Subgallate USP).
