
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
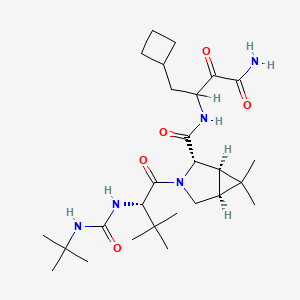

1. N-(3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl)-3-(2-((((1,1-dimethylethyl)amino)carbonyl)amino)-3,3-dimethyl-1-oxobutyl)-6,6-dimethyl-3-azabicyclo(3.1.0)hexan-2-carboxamide
2. Sch 503034
3. Sch-503034
4. Sch503034
5. Victrelis
1. 394730-60-0
2. Victrelis
3. Sch 503034
4. Ebp 520
5. Sch-503034
6. Sch503034
7. Ebp-520
8. 89bt58kelh
9. Chebi:68621
10. 3-{[(1r,2s,5s)-3-[(2s)-2-[(tert-butylcarbamoyl)amino]-3,3-dimethylbutanoyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2-yl]formamido}-4-cyclobutyl-2-oxobutanamide
11. (1r,2s,5s)-n-(4-amino-1-cyclobutyl-3,4-dioxobutan-2-yl)-3-((s)-2-(3-(tert-butyl)ureido)-3,3-dimethylbutanoyl)-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide
12. (1r,2s,5s)-n-(3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl)-3-((2s)-2-(((1,1-dimethylethyl)carbamoyl)amino)-3,3-dimethylbutanoyl)-6,6-dimethyl-3-azabicyclo(3.1.0)hexane-2-carboxamide
13. (1r,2s,5s)-n-(4-amino-1-cyclobutyl-3,4-dioxobutan-2-yl)-3-[(2s)-2-(tert-butylcarbamoylamino)-3,3-dimethylbutanoyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide
14. (1r,2s,5s)-n-(4-amino-1-cyclobutyl-3,4-dioxobutan-2-yl)-3-[n-(tert-butylcarbamoyl)-3-methyl-l-valyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide
15. (1s,4s,5r)-n-[3-amino-1-(cyclobutylmethyl)-2,3-dioxo-propyl]-3-[(2s)-2-(tert-butylcarbamoylamino)-3,3-dimethyl-butanoyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-4-carboxamide
16. Boceprevir [usan]
17. Boceprevir [usan:inn]
18. Unii-89bt58kelh
19. Hsdb 8081
20. Victrelis(tm)
21. Victrelis (tn)
22. 3-azabicyclo(3.1.0)hexane-2-carboxamide, N-(3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl)-3-((2s)-2-((((1,1-dimethylethyl)amino)carbonyl)amino)-3,3-dimethyl-1-oxobutyl)-6,6-dimethyl-, (1r,2s,5s)-
23. 3-azabicyclo[3.1.0]hexane-2-carboxamide, N-[3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[(2s)-2-[[[(1,1-dimethylethyl)amino]carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-, (1r,2s,5s)-
24. Boceprevir & Nm107
25. Boceprevir [mi]
26. Boceprevir [inn]
27. Boceprevir (inn/usan)
28. Boceprevir [vandf]
29. Boceprevir [mart.]
30. Boceprevir [who-dd]
31. Boceprevir [ema Epar]
32. Schembl640836
33. Chembl218394
34. Ebp520
35. Gtpl7876
36. Boceprevir [orange Book]
37. Bdbm12311
38. Dtxsid30960103
39. Bcp02502
40. Ex-a1336
41. Mfcd22208555
42. S3733
43. Akos005145787
44. Ebp-520;sch503034
45. Ccg-269852
46. Cs-0361
47. Db08873
48. Dt-0021
49. Ncgc00378631-01
50. Boc
51. Hy-10237
52. Sch 503034 & Nm107
53. D08876
54. Q410551
55. J-519910
56. (1r,5s)-n-[3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[2(s)-[[[(1,1-dimethylethyl)amino]carbonyl]amino]-3,3-dimethyl-1-oxobutyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexan-2(s)-carboxamide
57. 3-azabicyclo(3.1.0)hexane-2-carboxamide, N-(3-amino-1-(cyclobutylmethyl)-2,3-dioxopropyl)-3-((2s)-2-((((1,1- Dimethylethyl)amino)carbonyl)amino)-3,3-dimethyl-1-oxobutyl)-6,6- Dimethyl-, (1r,2s,5s)-
58. N-(4-amino-1-cyclobutyl-3,4-dioxobutan-2-yl)-3-[2-(tert-butylcarbamoylamino)-3,3-dimethylbutanoyl]-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide
59. N-(4-amino-1-cyclobutyl-3,4-dioxobutan-2-yl)-3-{n-[(tert-butylamino)(hydroxy)methylidene]-3-methylvalyl}-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboximidic Acid
| Molecular Weight | 519.7 g/mol |
|---|---|
| Molecular Formula | C27H45N5O5 |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 10 |
| Exact Mass | 519.34206955 g/mol |
| Monoisotopic Mass | 519.34206955 g/mol |
| Topological Polar Surface Area | 151 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 959 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Victrelis |
| PubMed Health | Boceprevir (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | VICTRELIS (boceprevir) is an inhibitor of the hepatitis C virus (HCV) non-structural protein 3 (NS3) serine protease.Boceprevir has the following chemical name: (1R,5S)-N-[3-Amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[2(S)-[[[(1,1-dimethylethyl)am... |
| Active Ingredient | Boceprevir |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 200mg |
| Market Status | Prescription |
| Company | Merck Sharp Dohme |
| 2 of 2 | |
|---|---|
| Drug Name | Victrelis |
| PubMed Health | Boceprevir (By mouth) |
| Drug Classes | Antiviral |
| Drug Label | VICTRELIS (boceprevir) is an inhibitor of the hepatitis C virus (HCV) non-structural protein 3 (NS3) serine protease.Boceprevir has the following chemical name: (1R,5S)-N-[3-Amino-1-(cyclobutylmethyl)-2,3-dioxopropyl]-3-[2(S)-[[[(1,1-dimethylethyl)am... |
| Active Ingredient | Boceprevir |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 200mg |
| Market Status | Prescription |
| Company | Merck Sharp Dohme |
Boceprevir is indicated for the treatment of chronic hepatitis C genotype 1 infection, in combination with peginterferon alfa and ribavirin, in adult patients (18 years and older) with compensated liver disease, including cirrhosis, who are previously untreated or who have failed previous interferon and ribavirin therapy. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for VICTRELIS (boceprevir) capsule (July 2012). Available from, as of November 13, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ae879ebe-b620-4829-b6f8-74b58da1c771
Boceprevir must not be used as monotherapy and should only be used in combination with peginterferon alfa and ribavirin. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for VICTRELIS (boceprevir) capsule (July 2012). Available from, as of November 13, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ae879ebe-b620-4829-b6f8-74b58da1c771
FDA notified healthcare professionals that the boceprevir (Victrelis) drug label has been revised to state that co-administration of boceprevir, a hepatitis C virus (HCV) protease inhibitor, along with certain ritonavir-boosted human immunodeficiency virus (HIV) protease inhibitors, is not recommended. The findings of a drug-drug interaction study and clinical trial showed that co-administration increased of the possibility of reducing the effectiveness of the medicines, permitting the amount of HCV or HIV virus in the blood to increase. Ritonavir-boosted HIV protease inhibitors include ritonavir-boosted atazanavir (Reyataz), ritonavir-boosted darunavir (Prezista), and lopinavir/ritonavir (Kaletra).
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
FDA notified healthcare professionals of updates to the prescribing information concerning interactions between protease inhibitors and certain statin drugs. Protease inhibitors and statins taken together may raise the blood levels of statins and increase the risk for muscle injury (myopathy). The most serious form of myopathy, called rhabdomyolysis, can damage the kidneys and lead to kidney failure, which can be fatal. BACKGROUND: Statins are a class of prescription drugs used together with diet and exercise to reduce blood levels of low-density lipoprotein (LDL) cholesterol ("bad cholesterol"). HIV protease inhibitors are a class of prescription anti-viral drugs used to treat HIV. HCV protease inhibitors are a class of prescription anti-viral drugs used to treat hepatitis C infection. RECOMMENDATION: Healthcare professionals should follow the recommendations in the prescribing information (drug labels) when prescribing HIV or HCV protease inhibitors with statins.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
FDA notified healthcare professionals and patients that drug interactions between the hepatitis C virus (HCV) protease inhibitor boceprevir (Victrelis) and certain ritonavir-boosted human immunodeficiency virus (HIV) protease inhibitors (atazanavir, lopinavir, darunavir) can potentially reduce the effectiveness of these medicines when they are used together. A drug interaction study showed that taking boceprevir (Victrelis) with ritonavir (Norvir) in combination with atazanavir (Reyataz) or darunavir (Prezista), or with Kaletra (lopinavir/ritonavir) reduced the blood levels of the HIV medicines and boceprevir in the body. FDA will be updating the boceprevir drug label to include information about these drug interactions. BACKGROUND: Boceprevir is a hepatitis C virus (HCV) protease inhibitor used with the medicines peginterferon alfa and ribavirin to treat chronic (long-lasting) hepatitis C infection in adults. HIV protease inhibitors are a class of anti-viral drugs used to treat HIV infection. Ritonavir is an HIV protease inhibitor used to "boost" other HIV protease inhibitors, increasing their levels in the blood and making them more effective. RECOMMENDATION: Patients should not stop taking any of their medicines without talking to their healthcare professional. Patients should contact their healthcare professional if they have any questions or concerns. Healthcare professionals who have started patients infected with both chronic HCV and HIV on boceprevir and antiretroviral therapy containing a ritonavir-boosted protease inhibitor should closely monitor patients for HCV treatment response and for potential HCV and HIV virologic rebound.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012
Because boceprevir must be used in conjunction with peginterferon alfa and ribavirin, it is contraindicated in women who are or may become pregnant and in male partners of pregnant women. The contraindications, warnings, and precautions for all 3 drugs should be considered.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 849
For more Drug Warnings (Complete) data for Boceprevir (21 total), please visit the HSDB record page.
Boceprevir, when used in combination with [DB00811], [DB00008], and [DB00022] is indicated for use in the treatment of chronic HCV genotype 1 infection in adults.
FDA Label
Victrelis is indicated for the treatment of chronic hepatitis-C (CHC) genotype-1 infection, in combination with peginterferon alfa and ribavirin, in adult patients with compensated liver disease who are previously untreated or who have failed previous therapy.
Boceprevir is classified as a direct-acting antiviral (DAA) and prevents viral replication in HCV genotype 1.
J05AE
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AP - Antivirals for treatment of hcv infections
J05AP03 - Boceprevir
Absorption
Boceprevir reaches peak plasma concentration 2 hours after administration. Absolute bioavailability has not been determined. When taken with food exposure increases up to 65%. In capsule, Boceprevir consists of two diaseromers in a 1:1 ratio. In plasma this ratio changes to 2:1 favoring the active diastereomer.
Route of Elimination
Boceprevir is mainly eliminated in the feces (79%) with a small amount eliminated in the urine (9%). Approximately 8% and 3% is excreted as the parent compound in the feces and urine respectively.
Volume of Distribution
The mean apparent volume of distribution for Bocepravir is 772 litres at steady state.
Clearance
Boceprevir has a mean total body clearance of 161 liters per hour.
In healthy subjects who received 800 mg three times daily alone, boceprevir drug exposure was characterized by AUC(T) of 5408 ng. hr per mL (n=71), Cmax of 1723 ng per mL (n=71), and Cmin of 88 ng per mL (n=71). Pharmacokinetic results were similar between healthy subjects and HCV-infected subjects.
US Natl Inst Health; DailyMed. Current Medication Information for VICTRELIS (boceprevir) capsule (July 2012). Available from, as of November 13, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ae879ebe-b620-4829-b6f8-74b58da1c771
Boceprevir was absorbed following oral administration with a median Tmax of 2 hours. Steady state AUC, Cmax, and Cmin increased in a less-than-dose-proportional manner and individual exposures overlapped substantially at 800 mg and 1200 mg, suggesting diminished absorption at higher doses. Accumulation is minimal (0.8- to 1.5-fold) and pharmacokinetic steady state is achieved after approximately 1 day of three times daily dosing.
US Natl Inst Health; DailyMed. Current Medication Information for VICTRELIS (boceprevir) capsule (July 2012). Available from, as of November 13, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ae879ebe-b620-4829-b6f8-74b58da1c771
Boceprevir should be administered with food. Food enhanced the exposure of boceprevir by up to 65% at the 800 mg three times daily dose, relative to the fasting state. The bioavailability of boceprevir was similar regardless of meal type (e.g., high-fat vs. low-fat) or whether taken 5 minutes prior to eating, during a meal, or immediately following completion of the meal. Therefore, boceprevir may be taken without regard to either meal type or timing of the meal.
US Natl Inst Health; DailyMed. Current Medication Information for VICTRELIS (boceprevir) capsule (July 2012). Available from, as of November 13, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ae879ebe-b620-4829-b6f8-74b58da1c771
Boceprevir has a mean apparent volume of distribution (Vd/F) of approximately 772 L at steady state in healthy subjects.
US Natl Inst Health; DailyMed. Current Medication Information for VICTRELIS (boceprevir) capsule (July 2012). Available from, as of November 13, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ae879ebe-b620-4829-b6f8-74b58da1c771
For more Absorption, Distribution and Excretion (Complete) data for Boceprevir (10 total), please visit the HSDB record page.
Bocepravir is primarily metabolized via the aldo-ketoreductase-mediated pathway producing a diastereomeric mix of metabolites at a 4 fold greater exposure than the parent compound. Boceprevir also undergoes oxidative metabolism via CYP3A4/5, although to a lesser extent.
Studies in vitro indicate that boceprevir primarily undergoes metabolism through the aldo-ketoreductase (AKR)-mediated pathway to ketone-reduced metabolites that are inactive against HCV. After a single 800-mg oral dose of (14)C-boceprevir, the most abundant circulating metabolites were a diasteriomeric mixture of ketone-reduced metabolites with a mean exposure approximately 4-fold greater than that of boceprevir. Boceprevir also undergoes, to a lesser extent, oxidative metabolism mediated by CYP3A4/5.
US Natl Inst Health; DailyMed. Current Medication Information for VICTRELIS (boceprevir) capsule (July 2012). Available from, as of November 13, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ae879ebe-b620-4829-b6f8-74b58da1c771
Boceprevir has a mean half-life of elimination of 3.4 hours.
Boceprevir is eliminated with a mean plasma half-life (t1/2) of approximately 3.4 hours.
US Natl Inst Health; DailyMed. Current Medication Information for VICTRELIS (boceprevir) capsule (July 2012). Available from, as of November 13, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ae879ebe-b620-4829-b6f8-74b58da1c771
Boceprevir is a NS3/4a protease inhibitor used to inhibit viral HCV replication. NS3/4a protease is an integral part of viral replication and mediates the cleavage the virally encoded polyprotein to mature proteins (NS4A, NS4B, NS5A and NS5B). Boceprevir covalently but reversibly binds the serine (S139) resiude in the active site via a ()-ketoamide functional group. This inhibits the proteolytic acitvity of the HCV 1a and 1b encoded enzyme.
Boceprevir is a selective hepatitis C virus (HCV) nonstructural (NS) 3/4A protease inhibitor. The drug is a direct-acting antiviral agent with activity against HCV. Boceprevir contains an alpha-ketoamide functional group that selectively, covalently, and reversibly binds the active serine site of HCV NS3 protease. By blocking proteolytic cleavage of NS4A, NS4B, NS5A, and NS5B from the HCV-encoded polyprotein, the drug inhibits HCV replication in host cells. Boceprevir has in vitro activity against HCV genotypes 1a and 1b, but is less active against genotypes 2, 2a, and 3a.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 851
Boceprevir is an inhibitor of the HCV NS3/4A protease that is necessary for the proteolytic cleavage of the HCV encoded polyprotein into mature forms of the NS4A, NS4B, NS5A and NS5B proteins. Boceprevir covalently, yet reversibly, binds to the NS3 protease active site serine (S139) through an (alpha)-ketoamide functional group to inhibit viral replication in HCV-infected host cells. In a biochemical assay, boceprevir inhibited the activity of recombinant HCV genotype 1a and 1b NS3/4A protease enzymes, with Ki values of 14 nM for each subtype.
US Natl Inst Health; DailyMed. Current Medication Information for VICTRELIS (boceprevir) capsule (July 2012). Available from, as of November 13, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ae879ebe-b620-4829-b6f8-74b58da1c771
... Boceprevir is a ketoamide protease inhibitor that binds reversibly to the HCV nonstructural NS3 protease active site inhibiting intracellular viral replication. Phase III clinical studies have demonstrated that, in combination with the current standard of care, boceprevir significantly increases the a sustained virological response rate in both treatment-naive and previously treated patients with genotype 1 CHC. ...
PMID:22397560 Trembling PM et al; Expert Rev Anti Infect Ther 10 (3): 269-79 (2012)
Related Excipient Companies

Excipients by Applications
Global Sales Information

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
64
PharmaCompass offers a list of Boceprevir API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Boceprevir manufacturer or Boceprevir supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Boceprevir manufacturer or Boceprevir supplier.
PharmaCompass also assists you with knowing the Boceprevir API Price utilized in the formulation of products. Boceprevir API Price is not always fixed or binding as the Boceprevir Price is obtained through a variety of data sources. The Boceprevir Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Boceprevir manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Boceprevir, including repackagers and relabelers. The FDA regulates Boceprevir manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Boceprevir API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Boceprevir supplier is an individual or a company that provides Boceprevir active pharmaceutical ingredient (API) or Boceprevir finished formulations upon request. The Boceprevir suppliers may include Boceprevir API manufacturers, exporters, distributors and traders.
click here to find a list of Boceprevir suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Boceprevir Drug Master File in Korea (Boceprevir KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Boceprevir. The MFDS reviews the Boceprevir KDMF as part of the drug registration process and uses the information provided in the Boceprevir KDMF to evaluate the safety and efficacy of the drug.
After submitting a Boceprevir KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Boceprevir API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Boceprevir suppliers with KDMF on PharmaCompass.
Boceprevir Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Boceprevir GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Boceprevir GMP manufacturer or Boceprevir GMP API supplier for your needs.
A Boceprevir CoA (Certificate of Analysis) is a formal document that attests to Boceprevir's compliance with Boceprevir specifications and serves as a tool for batch-level quality control.
Boceprevir CoA mostly includes findings from lab analyses of a specific batch. For each Boceprevir CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Boceprevir may be tested according to a variety of international standards, such as European Pharmacopoeia (Boceprevir EP), Boceprevir JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Boceprevir USP).
