

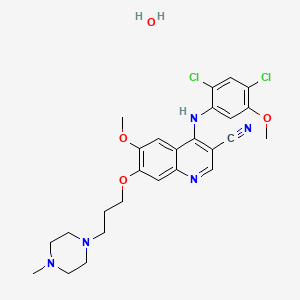

1. Bosutinib Hydrate
2. 918639-08-4
3. Ski-606 Monohydrate
4. Bosutinib.h2o
5. Bosutinib (hydrate)
6. 844zje6i55
7. Bosutinib Hydrate (jan)
8. 3-quinolinecarbonitrile, 4-((2,4-dichloro-5-methoxyphenyl)amino)-6-methoxy-7-(3-(4-methyl-1-piperazinyl)propoxy)-, Hydrate (1:1)
9. 4-(2,4-dichloro-5-methoxyanilino)-6-methoxy-7-[3-(4-methylpiperazin-1-yl)propoxy]quinoline-3-carbonitrile;hydrate
10. Bosutinib Hydrate [jan]
11. Bosutinib (as Monohydrate)
12. 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methylpiperazin-1-yl)propoxy]quinoline-3-carbonitrile Monohydrate
13. 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methylpiperazin-1-yl)propoxy]quinoline-3-carbonitrile--water (1/1)
14. Bosutinibhydrate
15. Unii-844zje6i55
16. Bosulif (tn)
17. Schembl2887945
18. Chebi:68533
19. Dtxsid20238722
20. Amy16532
21. Bcp17036
22. Hy-10158a
23. Akos027326604
24. Ac-30573
25. Bosutinib Monohydrate [orange Book]
26. Cs-0019907
27. Bosutinib (as Monohydrate) [ema Epar]
28. D09728
29. A900109
30. Q27137001
31. 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl) Propoxy]-3-quinolinecarbonitrile Monohydrate
32. 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl)propoxy]-3-quinolinecarbonitrile Monohydrate
| Molecular Weight | 548.5 g/mol |
|---|---|
| Molecular Formula | C26H31Cl2N5O4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 9 |
| Exact Mass | 547.1753099 g/mol |
| Monoisotopic Mass | 547.1753099 g/mol |
| Topological Polar Surface Area | 83.9 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 734 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Bosulif |
| PubMed Health | Bosutinib (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Active Ingredient | Bosutinib monohydrate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 100mg base; eq 500mg base |
| Market Status | Prescription |
| Company | Wyeth Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Bosulif |
| PubMed Health | Bosutinib (By mouth) |
| Drug Classes | Antineoplastic Agent |
| Active Ingredient | Bosutinib monohydrate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 100mg base; eq 500mg base |
| Market Status | Prescription |
| Company | Wyeth Pharms |
Bosulif is indicated for the treatment of adult patients with:
- newlydiagnosed chronic phase (CP) Philadelphia chromosome-positive chronic myelogenous leukaemia (Ph+ CML).
- CP, accelerated phase (AP), and blast phase (BP) Ph+ CML previously treated with one or more tyrosine kinase inhibitor(s) [TKI(s)] and for whom imatinib, nilotinib and dasatinib are not considered appropriate treatment options.

LOOKING FOR A SUPPLIER?