
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
API
0
FDF
0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
US Medicaid
NA
Annual Reports
NA
Finished Drug Prices
NA
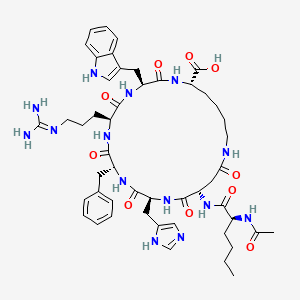

1. Pt-141
2. Vyleesi
1. 189691-06-3
2. Pt-141
3. Pt-141 Free Base
4. 6y24o4f92s
5. Vyleesi (tn)
6. (3s,6s,9r,12s,15s,23s)-15-[[(2s)-2-acetamidohexanoyl]amino]-9-benzyl-6-[3-(diaminomethylideneamino)propyl]-12-(1h-imidazol-5-ylmethyl)-3-(1h-indol-3-ylmethyl)-2,5,8,11,14,17-hexaoxo-1,4,7,10,13,18-hexazacyclotricosane-23-carboxylic Acid
7. (3s,6s,9r,12s,15s,23s)-12-((1h-imidazol-5-yl)methyl)-3-((1h-indol-3-yl)methyl)-15-((s)-2-acetamidohexanamido)-9-benzyl-6-(3-guanidinopropyl)-2,5,8,11,14,17-hexaoxo-1,4,7,10,13,18-hexaazacyclotricosane-23-carboxylic Acid
8. Bremelanotide [usan:inn]
9. Unii-6y24o4f92s
10. Bremelanotide, Pt-141
11. Bremelanotide [mi]
12. Bremelanotide [inn]
13. Pt-141 (bremelanotide)
14. Bremelanotide (usan/inn)
15. Bremelanotide [usan]
16. Bremelanotide [who-dd]
17. Chembl2070241
18. Schembl13574795
19. Schembl20337333
20. Gtpl10408
21. Dtxsid40893711
22. Chebi:177849
23. Pt141
24. 189691-06-3 (free Base)
25. Bdbm50389769
26. Akos005145807
27. Db11653
28. Hs-2024
29. Hy-18678
30. Cs-0013839
31. D06569
32. Q415353
33. Q-200747
34. (1z,3s,4z,6s,7z,9r,10e,12s,13z,15s,17e,23s)-12-((1h-imidazol-5-yl)methyl)-3-((1h-indol-3-yl)methyl)-9-benzyl-6-(3-guanidinopropyl)-2,5,8,11,14,17-hexahydroxy-15-(((s,z)-1-hydroxy-2-(((z)-1-hydroxyethylidene)amino)hexylidene)amino)-1,4,7,10,13,18-hexaazacyclotricosa-1,4,7,10,13,17-hexaene-23-carboxylic Acid
35. L-lysine, N-acetyl-l-norleucyl-l-.alpha.-aspartyl-l-histidyl-d-phenylalanyl-l-arginyl-l-tryptophyl-, (2->7)-lactam
36. L-lysine, N-acetyl-l-norleucyl-l-alpha-aspartyl-l-histidyl-d-phenylalanyl-l-arginyl-l-tryptophyl-, (2->7)-lactam
37. N-acetyl-l-2-aminohexanoyl-l-.alpha.-aspartyl-l-histidyl-d-phenylalanyl-l-arginyl-l-tryptophyl-l-lysine-(2->7)-lactam
38. N-acetyl-l-2-aminohexanoyl-l-alpha-aspartyl-l-histidyl-d-phenylalanyl-l-arginyl-l-tryptophyl-l-lysine-(2->7)-lactam
| Molecular Weight | 1025.2 g/mol |
|---|---|
| Molecular Formula | C50H68N14O10 |
| XLogP3 | 0.7 |
| Hydrogen Bond Donor Count | 13 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 17 |
| Exact Mass | 1024.52428442 g/mol |
| Monoisotopic Mass | 1024.52428442 g/mol |
| Topological Polar Surface Area | 379 Ų |
| Heavy Atom Count | 74 |
| Formal Charge | 0 |
| Complexity | 1950 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Bremelanotide is indicated to treat premenopausal women with hypoactive sexual desire disorder that is not due to a medical or psychiatric condition, problems with the relationship, or the effects of a medication or drug.
Bremelanotide is a melanocortin receptor agonist injected 45 minutes before anticipated sexual activity. Agonism of the melanocortin receptor MC1R also leads to increased melanin expression. Patients taking bremelanotide may also experience nausea, headache, and vomiting.
G - Genito urinary system and sex hormones
G02 - Other gynecologicals
G02C - Other gynecologicals
G02CX - Other gynecologicals
G02CX05 - Bremelanotide
Absorption
Bremelanotide has a Tmax or 1.0 hour (0.5-1.0 hours) and is 100% bioavailable. The Cmax is 72.8ng/mL and the AUC is 276hr\*ng/mL.
Route of Elimination
64.8% of a radiolabelled dose is excreted in the urine and 22.8% of the dose is recovered in the feces.
Volume of Distribution
The mean volume of distribution of bremelanotide is 25.05.8L.
Clearance
The mean clearance of bremelanotide is 6.51.0L/hr.
Bremelanotide is a 7 amino acid and so its metabolism consists of multiple hydrolysis reactions.
The half life of bremelanotide is 2.7 hours (1.9-4.0 hours).
Bremelanotide is an agonist of many melanocortin receptors which in order of potency are MC1R, MC4R, MC3R, MC5R, and MC2R. The mechanism by which agonism of these receptors translates to an improvement in hypoactive sexual desire disorder is currently unknown, however MC4R receptors are present in many areas of the central nervous system. MC3R and MC4R are found in the hypothalamus and are involved in food intake and energy homeostasis. One theory is that bremelanotide stimulates dopamine in the medial preoptic area, which is involved in the sexual behaviour of a number of organisms.
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vyleesi (bremelanotide) is a melanocortin receptor 4 agonist (MCR4), which is being evaluated in combination with tirzepatide for the treatment of obesity.
Lead Product(s): Bremelanotide Acetate,Tirzepatide
Therapeutic Area: Nutrition and Weight Loss Brand Name: Vyleesi
Study Phase: Phase IIProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 22, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bremelanotide Acetate,Tirzepatide
Therapeutic Area : Nutrition and Weight Loss
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Palatin Starts Phase 2 Study of Bremelanotide with Tirzepatide for Obesity
Details : Vyleesi (bremelanotide) is a melanocortin receptor 4 agonist (MCR4), which is being evaluated in combination with tirzepatide for the treatment of obesity.
Brand Name : Vyleesi
Molecule Type : Peptide
Upfront Cash : Not Applicable
August 22, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vyleesi (bremelanotide) is a melanocortin receptor 4 agonist (MCR4), which is being evaluated in combination with PDE5i for the treatment of erectile dysfunction.
Lead Product(s): Bremelanotide Acetate,Undisclosed
Therapeutic Area: Psychiatry/Psychology Brand Name: Vyleesi
Study Phase: Phase IIProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 20, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bremelanotide Acetate,Undisclosed
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Palatin Initiates Phase 2 Study of Bremelanotide for Erectile Dysfunction
Details : Vyleesi (bremelanotide) is a melanocortin receptor 4 agonist (MCR4), which is being evaluated in combination with PDE5i for the treatment of erectile dysfunction.
Brand Name : Vyleesi
Molecule Type : Peptide
Upfront Cash : Not Applicable
June 20, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vyleesi (bremelanotide) is a melanocortin receptor 4 agonist (MCR4), which is being evaluated in combination with tirzepatide for the treatment of obesity.
Lead Product(s): Bremelanotide Acetate,Tirzepatide
Therapeutic Area: Nutrition and Weight Loss Brand Name: Vyleesi
Study Phase: Phase IIProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable June 12, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bremelanotide Acetate,Tirzepatide
Therapeutic Area : Nutrition and Weight Loss
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Palatin Initiates Phase 2 Clinical Study of Bremelanotide for the Treatment of Obesity
Details : Vyleesi (bremelanotide) is a melanocortin receptor 4 agonist (MCR4), which is being evaluated in combination with tirzepatide for the treatment of obesity.
Brand Name : Vyleesi
Molecule Type : Peptide
Upfront Cash : Not Applicable
June 12, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
PT-141 (bremelanotide) is a melanocortin receptor 4 agonist (MCR4), which is being evaluated in combination with tirzepatide for the treatment of obesity.
Lead Product(s): Bremelanotide Acetate,Tirzepatide
Therapeutic Area: Nutrition and Weight Loss Brand Name: PT-141
Study Phase: Phase IProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable May 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bremelanotide Acetate,Tirzepatide
Therapeutic Area : Nutrition and Weight Loss
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Palatin Receives FDA Clearance for Bremelanotide with Tirzepatide in Obesity Treatment
Details : PT-141 (bremelanotide) is a melanocortin receptor 4 agonist (MCR4), which is being evaluated in combination with tirzepatide for the treatment of obesity.
Brand Name : PT-141
Molecule Type : Peptide
Upfront Cash : Not Applicable
May 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Cosette expands its women's health platform by acquiring Vyleesi (bremelanotide acetate), first as-needed treatment for premenopausal women with acquired generalized hypoactive sexual desire disorder.
Lead Product(s): Bremelanotide Acetate
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: Vyleesi
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Cosette Pharma
Deal Size: $171.0 million Upfront Cash: $12.0 million
Deal Type: Acquisition January 03, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bremelanotide Acetate
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Cosette Pharma
Deal Size : $171.0 million
Deal Type : Acquisition
Cosette Pharmaceuticals Acquires Vyleesi® from Palatin Technologies
Details : Cosette expands its women's health platform by acquiring Vyleesi (bremelanotide acetate), first as-needed treatment for premenopausal women with acquired generalized hypoactive sexual desire disorder.
Brand Name : Vyleesi
Molecule Type : Peptide
Upfront Cash : $12.0 million
January 03, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Cosette expands its women's health platform through divestment by adding Vyleesi, the first approved as-needed treatment for premenopausal women with acquired hypoactive sexual desire disorder.
Lead Product(s): Bremelanotide Acetate
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: Vyleesi
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Cosette Pharma
Deal Size: $171.0 million Upfront Cash: $12.0 million
Deal Type: Divestment January 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bremelanotide Acetate
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Cosette Pharma
Deal Size : $171.0 million
Deal Type : Divestment
Palatin Completes Sale of Vyleesi to Cosette Pharmaceuticals for up to $171 mn
Details : Cosette expands its women's health platform through divestment by adding Vyleesi, the first approved as-needed treatment for premenopausal women with acquired hypoactive sexual desire disorder.
Brand Name : Vyleesi
Molecule Type : Peptide
Upfront Cash : $12.0 million
January 02, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Through the divestment, Cosette will further expand its women's health platform by adding Vyleesi (bremelanotide acetate), the first and only as-needed treatment approved for premenopausal women with acquired, generalized hypoactive sexual desire disorder.
Lead Product(s): Bremelanotide Acetate
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: Vyleesi
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Cosette Pharma
Deal Size: $171.0 million Upfront Cash: $12.0 million
Deal Type: Divestment December 20, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bremelanotide Acetate
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Cosette Pharma
Deal Size : $171.0 million
Deal Type : Divestment
Palatin Completes Sale of Vyleesi® to Cosette Pharmaceuticals for up to $171 Million
Details : Through the divestment, Cosette will further expand its women's health platform by adding Vyleesi (bremelanotide acetate), the first and only as-needed treatment approved for premenopausal women with acquired, generalized hypoactive sexual desire disorde...
Brand Name : Vyleesi
Molecule Type : Peptide
Upfront Cash : $12.0 million
December 20, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vyleesi (bremelanotide injection) is a melanocortin receptor (MCR) agonist that is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder.
Lead Product(s): Bremelanotide Acetate
Therapeutic Area: Psychiatry/Psychology Brand Name: Vyleesi
Study Phase: Phase IIProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 10, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bremelanotide Acetate
Therapeutic Area : Psychiatry/Psychology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : Vyleesi (bremelanotide injection) is a melanocortin receptor (MCR) agonist that is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder.
Brand Name : Vyleesi
Molecule Type : Peptide
Upfront Cash : Not Applicable
August 10, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Vyleesi (bremelanotide injection) is a melanocortin receptor (MCR) agonist that is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder.
Lead Product(s): Bremelanotide Acetate
Therapeutic Area: Obstetrics/Gynecology (Women’s Health) Brand Name: Vyleesi
Study Phase: ApprovedProduct Type: Peptide
Sponsor: Fosun Pharmaceutical
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable August 08, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bremelanotide Acetate
Therapeutic Area : Obstetrics/Gynecology (Women’s Health)
Highest Development Status : Approved
Partner/Sponsor/Collaborator : Fosun Pharmaceutical
Deal Size : Not Applicable
Deal Type : Not Applicable
Palatin's Vyleesi® Licensee Fosun Pharma Reports First Commercial Sale in China and South Korean ...
Details : Vyleesi (bremelanotide injection) is a melanocortin receptor (MCR) agonist that is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder.
Brand Name : Vyleesi
Molecule Type : Peptide
Upfront Cash : Not Applicable
August 08, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
BMT-701 (bremelanotide) is a synthetic peptide analogue of melanocyte stimulating hormone alpha (MSHα), and its metabolism is by hydrolysis of amide bonds and digestion by cellular peptidases.
Lead Product(s): Bremelanotide Acetate
Therapeutic Area: Nephrology Brand Name: BMT-701
Study Phase: Phase IIProduct Type: Peptide
Sponsor: Not Applicable
Deal Size: Not Applicable Upfront Cash: Not Applicable
Deal Type: Not Applicable January 19, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Bremelanotide Acetate
Therapeutic Area : Nephrology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Not Applicable
Deal Size : Not Applicable
Deal Type : Not Applicable
Details : BMT-701 (bremelanotide) is a synthetic peptide analogue of melanocyte stimulating hormone alpha (MSHα), and its metabolism is by hydrolysis of amide bonds and digestion by cellular peptidases.
Brand Name : BMT-701
Molecule Type : Peptide
Upfront Cash : Not Applicable
January 19, 2023

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Patents & EXCLUSIVITIES
ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?