
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
JDMF
0
KDMF
0
NDC API
0
VMF
0
FDF
0
FDA Orange Book

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
EDQM
0
USP
0
JP
0
Others
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
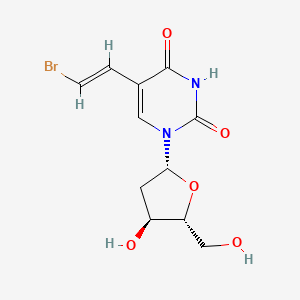

1. (e)-5-(2-bromovinyl)-2'-deoxyuridine
2. (z)-5-(2-bromovinyl)-2'-deoxyuridine
3. 5-(2-bromoethenyl)-2'-deoxyuridine
4. 5-(2-bromovinyl)-2'-deoxyuridine
5. 5-bvdu
6. Brivudin
7. E-5-(2-bromovinyl)-durd
8. Z-5-(2-bromovinyl)-durd
9. Zostex
1. 69304-47-8
2. Bvdu
3. (e)-5-(2-bromovinyl)-2'-deoxyuridine
4. Brivudin
5. Helpin
6. Zostex
7. Bromovinyldeoxyuridine
8. Brivudine [inn]
9. 5-bromovinyldeoxyuridine
10. (e)-5-(2-bromovinyl)-deoxyuridine
11. Rp101
12. Rp-101
13. Uridine, 5-[(1e)-2-bromoethenyl]-2'-deoxy-
14. Uridine, 5-(2-bromovinyl)-2'-deoxy-, (e)-
15. Brivudine (inn)
16. Nsc-633770
17. Ncgc00093656-01
18. 2m3055079h
19. 5-((e)-2-bromovinyl)-1-((2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidine-2,4(1h,3h)-dione
20. Brivudinum
21. Brivudina
22. Brivudinum [inn-latin]
23. Brivudina [inn-spanish]
24. Mfcd00058585
25. 5-[(e)-2-bromoethenyl]-1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2,3,4-tetrahydropyrimidine-2,4-dione
26. Bvd
27. Ccris 2831
28. 5-(2-bromovinyl)-2'-deoxyuridine
29. Nsc 633770
30. Trans-5-(2-bromovinyl)-2'-deoxyuridine
31. Bridic
32. Brivox
33. Zerpex
34. Brvdurd
35. Nsc633770
36. Unii-2m3055079h
37. Uridine, 5-(2-bromoethenyl)-2'-deoxy-, (e)-
38. Bv-durd
39. Zostex (tn)
40. (e)-5-(2-bromovinyl)-2-deoxyuridine
41. Brivudine [mi]
42. Brivudine [mart.]
43. Brivudine [who-dd]
44. Dsstox_cid_25755
45. Dsstox_rid_81098
46. Dsstox_gsid_45755
47. Lopac0_000175
48. Schembl99350
49. Chembl31634
50. Schembl141408
51. (e)-5-(2-bromovinyl)-durd
52. Dtxsid0045755
53. Zinc3653378
54. Tox21_111213
55. Bdbm50366681
56. S5009
57. Ua-618
58. Zb0745
59. E-5-(2-bromovinyl)-2'-deoxyuridine
60. Akos015833980
61. Ccg-204270
62. Cs-6292
63. Db03312
64. Lp00175
65. Sdccgsbi-0050163.p002
66. Ncgc00093656-02
67. 5-[(e)-2-bromoethenyl]-1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidine-2,4-dione
68. 5-[(e)-2-bromovinyl]-1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidine-2,4-dione
69. As-35234
70. Hy-13578
71. 5-[(e)-2-bromoethenyl]-2'-deoxyuridine
72. Cas-69304-47-8
73. A-176
74. B3404
75. Eu-0100175
76. B 9647
77. D07249
78. 5-((e)-2-bromovinyl)-2'-deoxyuridine
79. Q904107
80. Sr-01000075726
81. J-700153
82. Sr-01000075726-1
83. Bromovinyldeoxyuridine; 5-[(e)-2-bromoethenyl]-1-[(2r,4s,5r)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidine-2,4-dione
| Molecular Weight | 333.13 g/mol |
|---|---|
| Molecular Formula | C11H13BrN2O5 |
| XLogP3 | -0.4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 3 |
| Exact Mass | 332.00078 g/mol |
| Monoisotopic Mass | 332.00078 g/mol |
| Topological Polar Surface Area | 99.1 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 450 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AB - Nucleosides and nucleotides excl. reverse transcriptase inhibitors
J05AB15 - Brivudine
Global Sales Information

Market Place

ABOUT THIS PAGE


LOOKING FOR A SUPPLIER?