
Synopsis
Synopsis
0
USDMF
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Canada

0
Australia

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Lendorm
2. Lendormin
3. Lindormin
4. Sintonal
5. We 941
1. Lendormin
2. Lendorm
3. 57801-81-7
4. Mederantil
5. Sintonal
6. We 941
7. Nimbisan
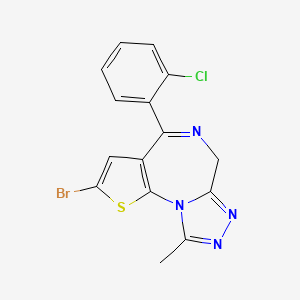
8. 2-bromo-4-(2-chlorophenyl)-9-methyl-6h-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine
9. We-941
10. 5xzm1r3dkf
11. Chembl32479
12. 2-bromo-4-(2-chlorophenyl)-9-methyl-6h-thieno(3,2-f)(1,2,4)triazolo(4,3-a)(1,4)diazepine
13. We-941-bs
14. 2-bromo-4-(o-chlorophenyl)-9-methyl-6h-thieno(3,2-f)-s-triazolo(4,3-a)(1,4)diazepine
15. Ncgc00183872-01
16. 6h-thieno(3,2-f)(1,2,4)triazolo(4,3-a)(1,4)diazepine, 2-bromo-4-(2-chlorophenyl)-9-methyl-
17. 6h-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine, 2-bromo-4-(2-chlorophenyl)-9-methyl-
18. Brotizolamum
19. Brotizolamum [inn-latin]
20. Einecs 260-964-5
21. Unii-5xzm1r3dkf
22. Brn 0839277
23. Lendormine
24. Indormyl
25. Ladormin
26. Brotizolam [usan:inn:ban:jan]
27. Lendormin (tn)
28. 2-bromo-4-(2-chlorophenyl)-9-methyl-6h-thieno-[3,2-f][1,2,4]-triazolo[4,3-a][1,4]diazepine
29. Brotizolam [mi]
30. Brotizolam [inn]
31. Brotizolam [jan]
32. Brotizolam [usan]
33. Dsstox_cid_2692
34. Brotizolam [mart.]
35. 8-bromo-6-(o-chlorophenyl)-1-methyl-4h-s-triazolo(3,4c)thieno(2,3e)-1,4-diazepine
36. Brotizolam [who-dd]
37. Dsstox_rid_76694
38. Dsstox_gsid_22692
39. Schembl44067
40. Brotizolam (jp17/usan/inn)
41. Dtxsid0022692
42. Chebi:31308
43. We 941-bs
44. Brotizolam [ep Monograph]
45. Brotizolam 0.1 Mg/ml In Methanol
46. Brotizolam 1.0 Mg/ml In Methanol
47. Zinc2570830
48. Tox21_113601
49. Bdbm50011875
50. Akos016014338
51. Db09017
52. Cas-57801-81-7
53. Db-053108
54. D01744
55. L000775
56. Q850074
57. 4-n-pentylphenyl-4-trans-n-propylcyclohexylcarboxylate
58. 2-bromo-4-(2-chloro-phenyl)-9-methyl-6h-1-thia-5,7,8,9a-tetraaza-cyclopenta[e]azulene
59. 2-bromo-4-(o-chlorophenyl)-9-methyl-6h-thieno[3,2-f]-s-triazolo[4,3-a]-[1,4]diazepine
60. 2-bromo-4-(2-chlorophenyl)-9-methyl-6h-thieno(3,2-f)-s-triazolo(4,3-a)(1,4)diazepine
61. 4-bromo-7-(2-chlorophenyl)-13-methyl-3-thia-1,8,11,12-tetrazatricyclo[8.3.0.02,6]trideca-2(6),4,7,10,12-pentaene
62. 8-bromo-6-(o-chlorophenyl)-1-methyl-4h-s-triazolo(3,4-c)thieno(2,3-e)-1,4-diazepine
| Molecular Weight | 393.7 g/mol |
|---|---|
| Molecular Formula | C15H10BrClN4S |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 391.94981 g/mol |
| Monoisotopic Mass | 391.94981 g/mol |
| Topological Polar Surface Area | 71.3 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 465 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Brotizolam is indicated for 2-4 weeks in the treatment of severe or debilitating insomnia.
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
N05CD09
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CD - Benzodiazepine derivatives
N05CD09 - Brotizolam
Absorption
The plasma concentration profile of brotizolam can be described as a one compartmental open model with first-order absorption.
Volume of Distribution
0.63 l/kg.
Clearance
Total clearance: 109 ml/min.
There are two primary metabolites: 1-methyl-hydroxy- and the 4-hydroxy-derivatives (Eberts et al., 1981; Boehringer Ingelheim, product information). The 4-hydroxymetabolites have a pharmacological activity which is far less than that of the parent drugs, but the 1-methyl-hydroxymetabolites probably have comparable activity (Gall et al., 1978; Jochemsen et al., 1982; Sethy & Harris, 1982; Jochemsen et al., unpublished results). These active compounds are, however, not present in plasma in measurable amounts following a single dose of brotizolam to young healthy subjects (Jochemsen et al., 1982; Jochemsen et al., unpublished results).
Brotizolam has known human metabolites that include 6-hydroxy-BRT and alpha-hydroxy-BRT.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
4.4 hours.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
22
PharmaCompass offers a list of Brotizolam API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Brotizolam manufacturer or Brotizolam supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Brotizolam manufacturer or Brotizolam supplier.
PharmaCompass also assists you with knowing the Brotizolam API Price utilized in the formulation of products. Brotizolam API Price is not always fixed or binding as the Brotizolam Price is obtained through a variety of data sources. The Brotizolam Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Brotizolam manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Brotizolam, including repackagers and relabelers. The FDA regulates Brotizolam manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Brotizolam API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Brotizolam manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Brotizolam supplier is an individual or a company that provides Brotizolam active pharmaceutical ingredient (API) or Brotizolam finished formulations upon request. The Brotizolam suppliers may include Brotizolam API manufacturers, exporters, distributors and traders.
click here to find a list of Brotizolam suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Brotizolam Drug Master File in Japan (Brotizolam JDMF) empowers Brotizolam API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Brotizolam JDMF during the approval evaluation for pharmaceutical products. At the time of Brotizolam JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Brotizolam suppliers with JDMF on PharmaCompass.
A Brotizolam CEP of the European Pharmacopoeia monograph is often referred to as a Brotizolam Certificate of Suitability (COS). The purpose of a Brotizolam CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Brotizolam EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Brotizolam to their clients by showing that a Brotizolam CEP has been issued for it. The manufacturer submits a Brotizolam CEP (COS) as part of the market authorization procedure, and it takes on the role of a Brotizolam CEP holder for the record. Additionally, the data presented in the Brotizolam CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Brotizolam DMF.
A Brotizolam CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Brotizolam CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Brotizolam suppliers with CEP (COS) on PharmaCompass.
A Brotizolam written confirmation (Brotizolam WC) is an official document issued by a regulatory agency to a Brotizolam manufacturer, verifying that the manufacturing facility of a Brotizolam active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Brotizolam APIs or Brotizolam finished pharmaceutical products to another nation, regulatory agencies frequently require a Brotizolam WC (written confirmation) as part of the regulatory process.
click here to find a list of Brotizolam suppliers with Written Confirmation (WC) on PharmaCompass.
Brotizolam Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Brotizolam GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Brotizolam GMP manufacturer or Brotizolam GMP API supplier for your needs.
A Brotizolam CoA (Certificate of Analysis) is a formal document that attests to Brotizolam's compliance with Brotizolam specifications and serves as a tool for batch-level quality control.
Brotizolam CoA mostly includes findings from lab analyses of a specific batch. For each Brotizolam CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Brotizolam may be tested according to a variety of international standards, such as European Pharmacopoeia (Brotizolam EP), Brotizolam JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Brotizolam USP).
