
Synopsis
Synopsis
0
VMF
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Busulfan Wellcome
2. Busulfex
3. Busulphan
4. Glyzophrol
5. Myelosan
6. Mylran
7. Mylecytan
8. Myleran
9. N-butane-1,3-di(methylsulfonate)
10. Wellcome, Busulfan
1. 55-98-1
2. Myleran
3. Busulphan
4. Sulphabutin
5. Leucosulfan
6. Busulfex
7. Myelosan
8. Citosulfan
9. Mielucin
10. Misulban
11. Mitostan
12. Myeloleukon
13. Mylecytan
14. Sulfabutin
15. Mablin
16. Mielevcin
17. Milecitan
18. Mielosan
19. Mileran
20. Butane-1,4-diyl Dimethanesulfonate
21. Buzulfan
22. 1,4-dimesyloxybutane
23. 1,4-butanediol Dimethanesulfonate
24. Myeleukon
25. Busulfanum
26. Myelosanum
27. 1,4-dimethanesulfonoxybutane
28. Tetramethylene Dimethane Sulfonate
29. 1,4-butanediol, Dimethanesulfonate
30. Busilvex
31. Busulfano
32. Busulphane
33. 1,4-dimethylsulfonyloxybutane
34. Nci-c01592
35. 1,4-dimethanesulfonyloxybutane
36. 1,4-bis(methanesulfonoxy)butane
37. 1,4-butanediol Dimethylsulfonate
38. 1,4-dimethylsulfonoxybutane
39. 1,4-bis(methanesulfonyloxy)butane
40. Gt 41
41. 4-methylsulfonyloxybutyl Methanesulfonate
42. Nsc-750
43. Cb 2041
44. 1,4-butanediol Dimethanesulphonate
45. An 33501
46. Methanesulfonic Acid, Tetramethylene Ester
47. Tetramethylene Bis(methanesulfonate)
48. 1,4-dimethanesulphonyloxybutane
49. Nsc 750
50. Tetramethylenester Kyseliny Methansulfonove
51. C.b. 2041
52. X 149
53. Gt 2041
54. Tetramethylene Bis[methanesulfonate]
55. 1,4-butanediol, Dimethanesulphonate
56. Nsc750
57. 1,4-bis[methanesulfonoxy]butane
58. Chebi:28901
59. 2041 C. B.
60. G.t. 41
61. G1ln9045dk
62. 4-(methanesulfonyloxy)butyl Methanesulfonate
63. Ncgc00090905-06
64. Mitosan
65. Dsstox_cid_910
66. Busulfanum [inn-latin]
67. Dsstox_rid_75857
68. Busulfano [inn-spanish]
69. Dsstox_gsid_20910
70. Methanesulfonic
71. Cas-55-98-1
72. Ccris 418
73. Sr-01000765405
74. Einecs 200-250-2
75. Brn 1791786
76. Unii-g1ln9045dk
77. Bisulfex
78. Ai3-25012
79. Busulfan;
80. Busulfan/myleran
81. Busulfan Solution
82. Busulfex Iv
83. Busulfan [usp:inn:ban:jan]
84. Hsdb 7605
85. Tetramethylenester Kyseliny Methansulfonove [czech]
86. Prestwick_989
87. Mfcd00007562
88. Tetramethylene {bis[methanesulfonate]}
89. Spectrum_000092
90. Busulfan [hsdb]
91. Busulfan [iarc]
92. Busulfan [inn]
93. Busulfan [jan]
94. 2041 C.b.
95. Busulfan [mi]
96. Busulfan [vandf]
97. Spectrum2_000067
98. Spectrum3_000320
99. Spectrum4_000259
100. Spectrum5_000928
101. Busulfan [mart.]
102. Busulfan [who-dd]
103. Busulfan [who-ip]
104. Chembl820
105. Ncimech_000192
106. Schembl4373
107. Busulfan [ema Epar]
108. Myelosanum [who-ip]
109. Wln: Ws1&o4osw1
110. Bspbio_001920
111. Kbiogr_000698
112. Kbioss_000512
113. Mls001076666
114. Busulfan (myleran, Busulfex)
115. Busulfan Fresenius Kabi
116. Divk1c_000847
117. Spectrum1500152
118. Busulfan (jp17/usp/inn)
119. Spbio_000253
120. Busulfan [ep Impurity]
121. Busulfan [orange Book]
122. Gtpl7136
123. Busulfan [ep Monograph]
124. Busulfan [usp Monograph]
125. Dtxsid3020910
126. Covzyzsdywqreu-uhfffaoysa-
127. Hms502k09
128. Kbio1_000847
129. Kbio2_000512
130. Kbio2_003080
131. Kbio2_005648
132. Kbio3_001420
133. Butane-1,4-diyldimethanesulfonate
134. Busulfanum [who-ip Latin]
135. Ninds_000847
136. Hms1920i07
137. Hms2091o09
138. Hms2233h04
139. Hms3259g15
140. Hms3370e11
141. Hms3655a21
142. Hms3712a20
143. Pharmakon1600-01500152
144. Amy33355
145. Hy-b0245
146. Zinc1530572
147. Tox21_111038
148. Tox21_201848
149. Tox21_300318
150. 1, {4-bis[methanesulfonoxy]butane}
151. 2041cb
152. Ac-198
153. Bdbm50237623
154. Ccg-35458
155. Nsc755916
156. S1692
157. Akos003614975
158. Tox21_111038_1
159. Db01008
160. Ks-5212
161. Nc00498
162. Nsc-755916
163. Idi1_000847
164. Ncgc00090905-01
165. Ncgc00090905-02
166. Ncgc00090905-03
167. Ncgc00090905-04
168. Ncgc00090905-05
169. Ncgc00090905-07
170. Ncgc00090905-08
171. Ncgc00090905-09
172. Ncgc00090905-10
173. Ncgc00090905-11
174. Ncgc00090905-12
175. Ncgc00254038-01
176. Ncgc00259397-01
177. Nci60_041640
178. Smr000058613
179. Tetramethylene Di(methanesulfonate)
180. Sbi-0051300.p003
181. Ft-0623291
182. Ft-0663910
183. Sw198555-3
184. C06862
185. D00248
186. D88731
187. 4-[(methylsulfonyl)oxy]butyl Methanesulfonate #
188. Ab00051929-10
189. Ab00051929-11
190. Ab00051929_12
191. Ab00051929_14
192. Busulfan, Analytical Standard, For Drug Analysis
193. Q348922
194. Sr-01000765405-2
195. Sr-01000765405-3
196. Sr-01000765405-7
197. Busulfan, European Pharmacopoeia (ep) Reference Standard
198. Z276508890
199. 129316-96-7
| Molecular Weight | 246.3 g/mol |
|---|---|
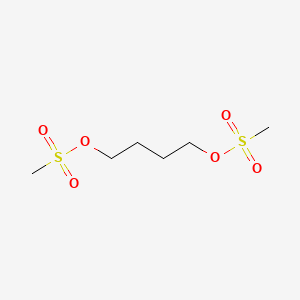
| Molecular Formula | C6H14O6S2 |
| XLogP3 | -0.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 246.02318051 g/mol |
| Monoisotopic Mass | 246.02318051 g/mol |
| Topological Polar Surface Area | 104 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 294 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Busulfex |
| PubMed Health | Busulfan (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Busulfan is a bifunctional alkylating agent known chemically as 1,4- butanediol, dimethanesulfonate. BUSULFEX (busulfan) Injection is intended for intravenous administration. It is supplied as a clear, colorless, sterile, solution in 10 mL single u... |
| Active Ingredient | Busulfan |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 6mg/ml |
| Market Status | Prescription |
| Company | Otsuka Pharm |
| 2 of 4 | |
|---|---|
| Drug Name | Myleran |
| PubMed Health | Busulfan |
| Drug Classes | Antineoplastic Agent |
| Drug Label | MYLERAN (busulfan) is a bifunctional alkylating agent. Busulfan is known chemically as 1,4-butanediol dimethanesulfonate and has the following structural formula:CH3SO2O(CH2)4OSO2CH3Busulfan is not a structural analog of the nitrogen mustards. MYLERA... |
| Active Ingredient | Busulfan |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2mg |
| Market Status | Prescription |
| Company | Aspen Global |
| 3 of 4 | |
|---|---|
| Drug Name | Busulfex |
| PubMed Health | Busulfan (Injection) |
| Drug Classes | Antineoplastic Agent |
| Drug Label | Busulfan is a bifunctional alkylating agent known chemically as 1,4- butanediol, dimethanesulfonate. BUSULFEX (busulfan) Injection is intended for intravenous administration. It is supplied as a clear, colorless, sterile, solution in 10 mL single u... |
| Active Ingredient | Busulfan |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 6mg/ml |
| Market Status | Prescription |
| Company | Otsuka Pharm |
| 4 of 4 | |
|---|---|
| Drug Name | Myleran |
| PubMed Health | Busulfan |
| Drug Classes | Antineoplastic Agent |
| Drug Label | MYLERAN (busulfan) is a bifunctional alkylating agent. Busulfan is known chemically as 1,4-butanediol dimethanesulfonate and has the following structural formula:CH3SO2O(CH2)4OSO2CH3Busulfan is not a structural analog of the nitrogen mustards. MYLERA... |
| Active Ingredient | Busulfan |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 2mg |
| Market Status | Prescription |
| Company | Aspen Global |
Busulfan is used in combination with cyclophosphamide as a conditioning regimen prior to allogeneic hematopoietic progenitor cell transplantation in patients with chronic myelogenous leukemia (CML) and is designated an orphan drug by the US Food and Drug Administration (FDA) for the treatment of this disease.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 951
/VET/ Antineoplastic agent used in adjunct therapy of acute granulocytic leukemias in small animals.
Milne, G.W.A. Veterinary Drugs: Synonyms and Properties. Ashgate Publishing Limited, Aldershot, Hampshire, England 2002., p. 93
Busulfan is an alkylating agent with myeloablative properties and activity against non-dividing marrow cells and, possibly, non-dividing malignant cells. Its use has been well established in the treatment of hematological malignancies, particularly in patients with chronic myeloid leukemia and other myeloproliferative syndromes.
European Medicines Agency (EMEA), Committee for Medicinal Products for Human Use; European Public Assessment Report (EPAR) (Science Discussion); Busilvex, p.3 (2005).https://www.emea.europa.eu/humandocs/PDFs/EPAR/busilvex/168603en6.pdf as of July 15, 2008
Busilvex followed by cyclophosphamide (BuCy4) or melphalan (BuMel) is indicated as conditioning treatment prior to conventional hematopoietic progenitor cell transplantation in pediatric patients.
European Medicines Agency (EMEA), Committee for Medicinal Products for Human Use; European Public Assessment Report (EPAR) (Annex I: Summary of Product Characteristics); Busilvex, p.2 (2005). Available from, as of July 15, 2008: https://www.emea.europa.eu/humandocs/PDFs/EPAR/busilvex/H-472-PI-en.pdf
Myleran is a potent drug. It should not be used unless a diagnosis of chronic myelogenous leukemia has been adequately established and the responsible physician is knowledgeable in assessing response to chemotherapy. Myleran can induce severe bone marrow hypoplasia. Reduce or discontinue the dosage immediately at the first sign of any unusual depression of bone marrow function as reflected by an abnormal decrease in any of the formed elements of the blood. A bone marrow examination should be performed if the bone marrow status is uncertain.
Thomson Health Care Inc.; Physicians' Desk Reference 62 ed., Montvale, NJ 2008, p. 1524
Life-threatening hepatic veno-occlusive disease has occurred in patients receiving busulfan (usually in combination with cyclophosphamide or other antineoplastic agents as a component of marrow-ablative therapy prior to bone marrow transplantation). The manufacturer states that a clear causal relationship to busulfan has not been demonstrated. Hepatic veno-occlusive disease diagnosed by clinical examination and laboratory findings occurred in 8% (5/61) of patients receiving IV busulfan in the allogeneic transplant clinical trial and was fatal in 40% (2/5) of cases. Overall mortality from hepatic veno-occlusive disease was 3% for the entire study population. Retrospectively, 3 of the 5 patients diagnosed with hepatic veno-occlusive disease were found to meet the Jones' criteria for this condition. In patients receiving high-dose oral busulfan as a component of a conditioning regimen prior to bone marrow transplant in randomized, controlled studies, the incidence of hepatic veno-occlusive disease was 7.7-12%.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 953
Interstitial pneumonitis and pulmonary fibrosis, which rarely were fatal, also have been reported in patients receiving high oral doses of busulfan as a component of a conditioning regimen prior to allogeneic bone marrow transplantation. Nonspecific interstitial fibrosis was diagnosed by lung biopsy in one patient receiving IV busulfan who subsequently died from respiratory failure.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 953
In patients receiving oral busulfan, pancytopenia generally occurs with failure to adequately monitor hematologic status and promptly discontinue the drug in response to a large or rapid decrease in leukocyte or platelet counts. Although individual variation in response to the drug does not appear to be an important contributing factor, some patients may be especially sensitive to busulfan and experience abrupt onset of neutropenia or thrombocytopenia. Busulfan-induced pancytopenia may be more prolonged than that induced by other alkylating agents; although recovery may take 1 month to 2 years, the toxicity is potentially reversible and patients should be vigorously supported through any period of severe pancytopenia. Some patients develop bone marrow fibrosis or chronic aplasia which is probably due to busulfan toxicity. Aplastic anemia, sometimes irreversible, has been reported rarely in patients receiving oral busulfan; aplastic anemia usually has occurred following high doses of the drug or long-term administration of conventional doses.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 952
For more Drug Warnings (Complete) data for BUSULFAN (42 total), please visit the HSDB record page.
For use in combination with cyclophosphamide as a conditioning regimen prior to allogeneic hematopoietic progenitor cell transplantation for chronic myelogenous (myeloid, myelocytic, granulocytic) leukemia (FDA has designated busulfan as an orphan drug for this use). It is also used as a component of pretransplant conditioning regimens in patients undergoing bone marrow transplantation for acute myeloid leukemia and nonmalignant diseases.
FDA Label
Busulfan Fresenius Kabi followed by cyclophosphamide (BuCy2) is indicated as conditioning treatment prior to conventional haematopoietic progenitor cell transplantation (HPCT) in adult patients when the combination is considered the best available option.
Busulfan Fresenius Kabi followed by cyclophosphamide (BuCy4) or melphalan (BuMel) is indicated as conditioning treatment prior to conventional haematopoietic progenitor cell transplantation in paediatric patients.
Busilvex followed by cyclophosphamide (BuCy2) is indicated as conditioning treatment prior to conventional haematopoietic progenitor cell transplantation (HPCT) in adult patients when the combination is considered the best available option.
Busilvex following fludarabine (FB) is indicated as conditioning treatment prior to haematopoietic progenitor cell transplantation (HPCT) in adult patients who are candidates for a reduced-intensity conditioning (RIC) regimen.
Busilvex followed by cyclophosphamide (BuCy4) or melphalan (BuMel) is indicated as conditioning treatment prior to conventional haematopoietic progenitor cell transplantation in paediatric patients.
Busulfan is an antineoplastic in the class of alkylating agents and is used to treat various forms of cancer. Alkylating agents are so named because of their ability to add alkyl groups to many electronegative groups under conditions present in cells. They stop tumor growth by cross-linking guanine bases in DNA double-helix strands - directly attacking DNA. This makes the strands unable to uncoil and separate. As this is necessary in DNA replication, the cells can no longer divide. In addition, these drugs add methyl or other alkyl groups onto molecules where they do not belong which in turn leads to a miscoding of DNA. Alkylating agents are cell cycle-nonspecific and work by three different mechanisms, all of which achieve the same end result - disruption of DNA function and cell death. Overexpression of MGST2, a glutathione s-transferase, is thought to confer resistance to busulfan. The role of MGST2 in the metabolism of busulfan is unknown however.
Antineoplastic Agents, Alkylating
A class of drugs that differs from other alkylating agents used clinically in that they are monofunctional and thus unable to cross-link cellular macromolecules. Among their common properties are a requirement for metabolic activation to intermediates with antitumor efficacy and the presence in their chemical structures of N-methyl groups, that after metabolism, can covalently modify cellular DNA. The precise mechanisms by which each of these drugs acts to kill tumor cells are not completely understood. (From AMA, Drug Evaluations Annual, 1994, p2026) (See all compounds classified as Antineoplastic Agents, Alkylating.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)
Myeloablative Agonists
Agents that destroy bone marrow activity. They are used to prepare patients for BONE MARROW TRANSPLANTATION or STEM CELL TRANSPLANTATION. (See all compounds classified as Myeloablative Agonists.)
Alkylating Agents
Highly reactive chemicals that introduce alkyl radicals into biologically active molecules and thereby prevent their proper functioning. Many are used as antineoplastic agents, but most are very toxic, with carcinogenic, mutagenic, teratogenic, and immunosuppressant actions. They have also been used as components in poison gases. (See all compounds classified as Alkylating Agents.)
L01AB01
L01AB01
L01AB01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
L - Antineoplastic and immunomodulating agents
L01 - Antineoplastic agents
L01A - Alkylating agents
L01AB - Alkyl sulfonates
L01AB01 - Busulfan
Absorption
Completely absorbed from the gastrointestinal tract. Busulfan is a small, highly lipophilic molecule that crosses the blood-brain-barrier. The absolute bioavailability, if a single 2 mg IV bolus injection is given to adult patients, is 80% 20%. In children (1.5 - 6 years old), the absolute bioavailability was 68% 31%. When a single oral dose is given to patients, the area under the curve (AUC) was 130 nghr/mL. The peak plasma concentration when given orally is 30 ng/mL (after dose normalization to 2 mg). It takes 0.9 hours to reach peak plasma concentration after dose normalization to 4 mg.
Route of Elimination
Following administration of 14C- labeled busulfan to humans, approximately 30% of the radioactivity was excreted into the urine over 48 hours; negligible amounts were recovered in feces. Less than 2% of the administered dose is excreted in the urine unchanged within 24 hours. Elimination of busulfan is independent of renal function.
Clearance
2.52 ml/min/kg [Following an infusion of dose of 0.8 mg/kg every six hours, for a total of 16 doses over four days]
The pharmacokinetic disposition of busulfan differs in children versus adults. The mean bioavailability of busulfan is lower in children than in adults; the interindividual variation in bioavailability for oral busulfan is large, particularly in children. In a pharmacokinetic study in children receiving IV busulfan (0.8 or 1 mg/kg based on actual body weight), an estimated volume of distribution of 0.64 L/kg (with an interpatient variability of 11%) was reported.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 956
Busulfan, a small and highly lipophilic molecule, easily crosses the blood-brain barrier. Busulfan concentrations in the cerebrospinal fluid (CSF) are approximately equal to concurrent busulfan plasma concentrations. It is not known whether the drug is distributed into milk.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 956
For adults receiving busulfan 2, 4, or 6 mg orally as a single dose on consecutive days, the drug exhibits linear kinetics for both the maximum plasma concentration and the area under the concentration-time curve (AUC); a mean peak plasma concentration (normalized to a dose of 2 mg) of about 30 ng/mL was observed. In a study of 12 patients receiving single oral busulfan doses of 4-8 mg, a mean peak plasma concentration (normalized to a dose of 4 mg) of about 68 ng/mL was reported; the time to peak plasma concentration was about 0.9 hours.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 956
Busulfan is rapidly and completely absorbed from the GI tract after oral administration of the drug. The effect of food on the bioavailability of busulfan is not known.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 956
For more Absorption, Distribution and Excretion (Complete) data for BUSULFAN (11 total), please visit the HSDB record page.
Busulfan is extensively metabolizes in the hepatic. Busulfan is predominantly metabolized by conjugation with glutathione, both spontaneously and by glutathione S-transferase (GST) catalysis. GSTA1 is the primary GST isoform that facilitates the the metabolism of busulfan. Other GST isoforms that are also involved are GSTM1 and GSTP1. At least 12 metabolites have been identified among which tetrahydrothiophene, tetrahydrothiophene 12-oxide, sulfolane, and 3-hydroxysulfolane were identified. These metabolites do not have cytotoxic activity.
After IP injections of 2:3-(14)C-Myleran in the rat, rabbit and mouse, 60% of the urinary radioactivity was found to be in the form of the 3-hydroxy tetrahydrothiophene-1,1-dioxide, a sulphone. It is suggested that in vivo Myleran undergoes a reaction with cysteine or a cysteinyl moiety to form a cyclic sulphonium ion, which in turn undergoes cleavage to the tetrahydrothiophene, oxidation to the 1,1-dioxide and biological hydroxylation to the 3-hydroxy compound.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V4 250 (1974)
In the rat and mouse 50-60% of a single dose of Myleran-(35)S (10 mg/kg bw) injected intraperitoneally in arachis oil was excreted within 24 to 48 hours, mainly as methane sulphonic acid; a small amount of unchanged Myleran and two unidentified components were present. In the rabbit, methane sulphonic acid was the only metabolite found in the urine.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V4 250 (1974)
The metabolic fate of busulfan has been studied in rats and humans using (14)C- and (35)S-labeled materials. In humans, as in the rat, almost all of the radioactivity in (35)S-labeled busulfan is excreted in the urine in the form of (35)S-methanesulfonic acid. /It was/ demonstrated that the formation of methanesulfonic acid in vivo in the rat is not due to a simple hydrolysis of busulfan to 1,4-butanediol, since only about 4% of 2,3-(14)C-busulfan was excreted as carbon dioxide, whereas 2,3-(14)C-1,4-butanediol was converted almost exclusively to carbon dioxide. The predominant reaction of busulfan in the rat is the alkylation of sulfhydryl groups (particularly cysteine and cysteine-containing compounds) to produce a cyclic sulfonium compound which is the precursor of the major urinary metabolite of the 4-carbon portion of the molecule, 3-hydroxytetrahydrothiophene-1,1-dioxide. This has been termed a "sulfur-stripping" action of busulfan and it may modify the function of certain sulfur-containing amino acids, polypeptides, and proteins; whether this action makes an important contribution to the cytotoxicity of busulfan is unknown.
Thomson Health Care Inc.; Physicians' Desk Reference 62 ed., Montvale, NJ 2008, p. 1525
(14)C busulfan was administered ip (15 mg/kg) to 5 male Sprague-Dawley rats. For 72 hours, the urinary recovery of (14)C was approximately 70% of the total dose, while the fecal excretion was within the range 1.5-2%. The pattern of the urinary metabolites of busulfan was studied by HPLC in combination with radioactivity detection of the pooled urine. At least eight radioactive fractions could be separated. Three major metabolite peaks were identified by GC/MS and NMR spectroscopy: 3-hydroxysulfolane (39% of total urine radioactivity), tetrahydrothiophene 1- oxide (20%), and sulfolane (13%). Busulfan (6%) and tetrahydrofuran (2%) were also identified. A sulfonium ion glutathione conjugate was hypothesised as another metabolite, but was not isolated because it was very unstable. However, another compound was observed. This metabolite co-eluted with the sulfonium ion obtained of the reaction of busulfan with N-acetyl-L-cysteine and produced tetrahydrothiophene when hydrolyzed. Finally, busulfan and the three main metabolites were tested for cytotoxicity on Chinese V79 hamster cells in vitro. Cell toxicity was induced only by busulfan, which indicates that the cytotoxicity in vivo is mediated by the parent compound, as expected.
European Medicines Agency (EMEA), Committee for Medicinal Products for Human Use; European Public Assessment Report (EPAR) (Science Discussion); Busilvex, p.10 (2005). Available from, as of July 15, 2008: https://www.emea.europa.eu/humandocs/PDFs/EPAR/busilvex/168603en6.pdf
2.6 hours
The terminal half life /in children from < 6 months up to 17 years old/ ranged from 2.26 to 2.52 hr.
European Medicines Agency (EMEA), Committee for Medicinal Products for Human Use; European Public Assessment Report (EPAR) (Annex I: Summary of Product Characteristics); Busilvex, p.12 (2005). Available from, as of July 15, 2008: https://www.emea.europa.eu/humandocs/PDFs/EPAR/busilvex/H-472-PI-en.pdf
The elimination half-life is about 2.6 hours in adults receiving oral busulfan.
American Society of Health System Pharmacists. AHFS Drug Information 2008. Bethesda, Maryland 2008, p. 956
Following intravenous administration, the mean half life of busulfan ranged from 2.83 hours to 3.90 hours ... . Oral busulfan had a mean half life of 3.87 hours.
European Medicines Agency (EMEA), Committee for Medicinal Products for Human Use; European Public Assessment Report (EPAR) (Science Discussion); Busilvex, p.15 (2005). Available from, as of July 15, 2008: https://www.emea.europa.eu/humandocs/PDFs/EPAR/busilvex/168603en6.pdf
Busulfan is an alkylating agent that contains 2 labile methanesulfonate groups attached to opposite ends of a 4-carbon alkyl chain. Once busulfan is hydrolyzed, the methanesulfonate groups are released and carbonium ions are produced. These carbonium ions alkylate DNA, which results in the interference of DNA replication and RNA transcription, ultimately leading to the disruption of nucleic acid function. Specifically, its mechanism of action through alkylation produces guanine-adenine intrastrand crosslinks. These crosslinks occur through a SN2 reaction guanine N7 nucleophilically attacks the carbon adjacent to the mesylate leaving group. This kind of damage cannot be repaired by cellular machinery and thus the cell undergoes apoptosis.
The primary molecular action of busulfan is alkylation of intracellular nucleophiles. Both proteins and nucleic acids are affected. With regard to DNA, busulfan reacts with guanine residues to form a fourcarbon di-guanine DNA cross-linkage with release of methylsulfonate. The DNA cross-linkage causes misreading of the DNA code and single-strand breakage. The degree of DNA cross-linkage has been shown to be proportional to the dose and cytotoxicity of the compound. Busulfan-induced crosslinkages of DNA to nuclear proteins may also occur and is considered a cytotoxic mechanism. Busulfan has also been reported to esterify phosphate groups of chromosomal DNA, accounting for the fragmentation of chromosomes seen in various cell types after treatment. Chromosomal damage further contributes to the overall cytotoxic effect.
European Medicines Agency (EMEA), Committee for Medicinal Products for Human Use; European Public Assessment Report (EPAR) (Science Discussion); Busilvex, p.6 (2005). Available from, as of July 15, 2008: https://www.emea.europa.eu/humandocs/PDFs/EPAR/busilvex/168603en6.pdf
Studies of cloned tumour and normal cells indicate that busulfan interrupts certain parts of the cell mitotic cycle. While busulfan can act on cells at any stage of mitosis, similar to other alkylating agents, in vitro studies with tumour cell lines indicate that replicating cells are most sensitive to busulfan in the late G1 phase and that progression through the cell cycle is blocked in the G2 phase. Cultured cells in the S-phase, which involves DNA synthesis, are not as affected because the DNA repair systems are active at this stage. Clinically, busulfan is cytotoxic to proliferating tissues, especially granulopoietic cells in the bone marrow.
European Medicines Agency (EMEA), Committee for Medicinal Products for Human Use; European Public Assessment Report (EPAR) (Science Discussion); Busilvex, p.6 (2005). Available from, as of July 15, 2008: https://www.emea.europa.eu/humandocs/PDFs/EPAR/busilvex/168603en6.pdf

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
70
PharmaCompass offers a list of Busulfan API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Busulfan manufacturer or Busulfan supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Busulfan manufacturer or Busulfan supplier.
PharmaCompass also assists you with knowing the Busulfan API Price utilized in the formulation of products. Busulfan API Price is not always fixed or binding as the Busulfan Price is obtained through a variety of data sources. The Busulfan Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Busulfan manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Busulfan, including repackagers and relabelers. The FDA regulates Busulfan manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Busulfan API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Busulfan manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Busulfan supplier is an individual or a company that provides Busulfan active pharmaceutical ingredient (API) or Busulfan finished formulations upon request. The Busulfan suppliers may include Busulfan API manufacturers, exporters, distributors and traders.
click here to find a list of Busulfan suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Busulfan DMF (Drug Master File) is a document detailing the whole manufacturing process of Busulfan active pharmaceutical ingredient (API) in detail. Different forms of Busulfan DMFs exist exist since differing nations have different regulations, such as Busulfan USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Busulfan DMF submitted to regulatory agencies in the US is known as a USDMF. Busulfan USDMF includes data on Busulfan's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Busulfan USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Busulfan suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Busulfan Drug Master File in Japan (Busulfan JDMF) empowers Busulfan API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Busulfan JDMF during the approval evaluation for pharmaceutical products. At the time of Busulfan JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Busulfan suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Busulfan Drug Master File in Korea (Busulfan KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Busulfan. The MFDS reviews the Busulfan KDMF as part of the drug registration process and uses the information provided in the Busulfan KDMF to evaluate the safety and efficacy of the drug.
After submitting a Busulfan KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Busulfan API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Busulfan suppliers with KDMF on PharmaCompass.
A Busulfan CEP of the European Pharmacopoeia monograph is often referred to as a Busulfan Certificate of Suitability (COS). The purpose of a Busulfan CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Busulfan EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Busulfan to their clients by showing that a Busulfan CEP has been issued for it. The manufacturer submits a Busulfan CEP (COS) as part of the market authorization procedure, and it takes on the role of a Busulfan CEP holder for the record. Additionally, the data presented in the Busulfan CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Busulfan DMF.
A Busulfan CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Busulfan CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Busulfan suppliers with CEP (COS) on PharmaCompass.
A Busulfan written confirmation (Busulfan WC) is an official document issued by a regulatory agency to a Busulfan manufacturer, verifying that the manufacturing facility of a Busulfan active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Busulfan APIs or Busulfan finished pharmaceutical products to another nation, regulatory agencies frequently require a Busulfan WC (written confirmation) as part of the regulatory process.
click here to find a list of Busulfan suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Busulfan as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Busulfan API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Busulfan as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Busulfan and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Busulfan NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Busulfan suppliers with NDC on PharmaCompass.
Busulfan Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Busulfan GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Busulfan GMP manufacturer or Busulfan GMP API supplier for your needs.
A Busulfan CoA (Certificate of Analysis) is a formal document that attests to Busulfan's compliance with Busulfan specifications and serves as a tool for batch-level quality control.
Busulfan CoA mostly includes findings from lab analyses of a specific batch. For each Busulfan CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Busulfan may be tested according to a variety of international standards, such as European Pharmacopoeia (Busulfan EP), Busulfan JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Busulfan USP).
