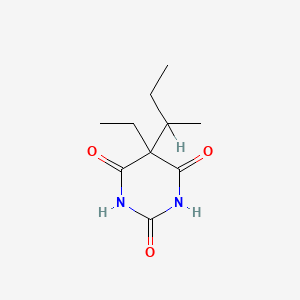

1. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-ethyl-5-(1-methylpropyl)-, Monosodium Salt
2. Butabarbital Sodium
3. Butabarbitone
4. Butisol
5. Butisol Sodium
6. Sarisol
7. Secbutabarbital
8. Secbutabarbital Sodium
9. Secbutobarbitone
10. Secumalum
1. Secbutabarbital
2. Butatab
3. Secbutobarbitone
4. Butabarbitone
5. Secbubarbital
6. Medarsed
7. Unicelles
8. Butisol
9. Nilox
10. Butabarb
11. Butrate
12. Butatal
13. Sec-butobarbitone
14. 125-40-6
15. Buticaps
16. 5-sec-butyl-5-ethylbarbituric Acid
17. 5-ethyl-5-(1-methylpropyl)barbituric Acid
18. 5-sec-butyl-5-ethylmalonyl Urea
19. Barbituric Acid, 5-sec-butyl-5-ethyl-
20. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-ethyl-5-(1-methylpropyl)-
21. Sodium Butabarbital
22. Butabarbital Ciii
23. Nsc 27517
24. Butabarbital (usp)
25. Butabarbital [usp]
26. 5-ethyl-5-(1-methylpropyl)-2,4,6(1h,3h,5h)-pyrimidinetrione
27. Nsc27517
28. 5-sec-butyl-5-ethyl-2,4,6(1h,3h,5h)-pyrimidinetrione
29. Chebi:3228
30. P0078o25a9
31. 5-sec-butyl-5-ethylpyrimidine-2,4,6(1h,3h,5h)-trione
32. Butabarbital (van)
33. Secbutobarbital
34. Secbutabarbitale
35. Secbutabarbitalum
36. Secbutabarbitale [dcit]
37. Secbutabarbitalum [inn-latin]
38. Secbutabarbital [inn]
39. 5-ethyl-5-(1-methylpropyl)barbiturate
40. Hsdb 3018
41. Wln: T6vmvmv Fhj Fy2 & 1 F2
42. Einecs 204-738-6
43. Brn 0199127
44. 5-butan-2-yl-5-ethyl-1,3-diazinane-2,4,6-trione
45. Unii-p0078o25a9
46. Secbutabarbital (ban)
47. Butabarbital [mi]
48. 5-sec-butyl-5-ethyl-barbituric Acid
49. Pyridium Plus (salt/mix)
50. Chembl449
51. Butabarbital [hsdb]
52. Secbutabarbital [inn:ban]
53. Butabarbital [vandf]
54. Schembl79254
55. Gtpl7137
56. Secbutabarbital [mart.]
57. 5-(butan-2-yl)-5-ethyl-1,3-diazinane-2,4,6-trione
58. Dtxsid2022709
59. Schembl22556204
60. Secbutabarbital [who-dd]
61. 5-(butan-2-yl)-5-ethylpyrimidine-2,4,6(1h,3h,5h)-trione
62. Butabarbital [usp Impurity]
63. Butabarbital Ciii [usp-rs]
64. 5-ethyl-5-(1-methylpropyl)pyrimidine-2,4,6(1h,3h,5h)-trione
65. Butabarbital [usp Monograph]
66. Hy-u00167
67. Nsc-27517
68. Akos015962208
69. Cs-7224
70. Db00237
71. Ac-16078
72. C07827
73. D03180
74. Q410608
75. 5-sec-butyl-5-ethyl-2,4,6(1h,3h,5h)-pyrimidinetrione #
76. 2,6(1h,3h,5h)-pyrimidinetrione, 5-ethyl-5-(1-methylpropyl)-
77. 5-(butan-2-yl)-5-ethyl-4,6-dihydroxy-2,5-dihydropyrimidin-2-one
78. Butabarbital Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
| Molecular Weight | 212.25 g/mol |
|---|---|
| Molecular Formula | C10H16N2O3 |
| XLogP3 | 1.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 212.11609238 g/mol |
| Monoisotopic Mass | 212.11609238 g/mol |
| Topological Polar Surface Area | 75.3 Ų |
| Heavy Atom Count | 15 |
| Formal Charge | 0 |
| Complexity | 292 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Butisol sodium |
| PubMed Health | Butabarbital (By mouth) |
| Drug Classes | Sedative |
| Drug Label | BUTISOL SODIUM (butabarbital sodium tablets, USP and butabarbital sodium oral solution, USP) is a non-selective central nervous system depressant which is used as a sedative or hypnotic. It is available for oral administration as Tablets containing... |
| Active Ingredient | Butabarbital sodium |
| Dosage Form | Elixir; Tablet |
| Route | Oral |
| Strength | 30mg/5ml; 50mg; 30mg |
| Market Status | Prescription |
| Company | Meda Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Butisol sodium |
| PubMed Health | Butabarbital (By mouth) |
| Drug Classes | Sedative |
| Drug Label | BUTISOL SODIUM (butabarbital sodium tablets, USP and butabarbital sodium oral solution, USP) is a non-selective central nervous system depressant which is used as a sedative or hypnotic. It is available for oral administration as Tablets containing... |
| Active Ingredient | Butabarbital sodium |
| Dosage Form | Elixir; Tablet |
| Route | Oral |
| Strength | 30mg/5ml; 50mg; 30mg |
| Market Status | Prescription |
| Company | Meda Pharms |
Adjuvants, Anesthesia; GABA Modulators; Sedatives, Barbiturate
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Butabarbital is indicated for use as a sedative or hypnotic. /Included in US product label/
US Natl Inst Health; DailyMed. Current Medication Information for BUTISOL SODIUM (butabarbital sodium tablets) (July 2009). Available from, as of March 7, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10579
Butabarbital sodium is used for routine sedation and to relieve anxiety and provide sedation postoperatively. ... Also used as a hypnotic in the short-term treatment of insomnia for periods up to 2 weeks in duration. /Butabarbital sodium/
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 92. Bethesda, MD: American Society of Hospital Pharmacists, Inc., 1992 (Plus Supplements 1992)., p. 1323
Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated.
US Natl Inst Health; DailyMed. Current Medication Information for BUTISOL SODIUM TABLETS (butabarbital sodium tablets) (July 2009). Available from, as of March 7, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10579
Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequences of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative-hypnotic drugs. Because some of the important adverse effects of sedative-hypnotics appear to be dose related, it is important to use the smallest possible effective dose, especially in the elderly.
US Natl Inst Health; DailyMed. Current Medication Information for BUTISOL SODIUM TABLETS (butabarbital sodium tablets) (July 2009). Available from, as of March 7, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10579
Complex behaviors such as "sleep driving" (ie, driving while not fully awake after ingestion of a sedative-hypnotic, with amnesia for the event) have been reported. These events can occur in sedative-hypnotic-naive as well as in sedative-hypnotic-experienced persons. Although behaviors such as sleep-driving may occur with sedative-hypnotics alone at therapeutic doses, the use of alcohol and other CNS depressants with sedative-hypnotics appears to increase the risk of such behaviors, as does the use of sedative-hypnotics at doses exceeding the maximum recommended dose. Due to the risk to the patient and the community, discontinuation of sedative-hypnotics should be strongly considered for patients who report a "sleep driving" episode".
US Natl Inst Health; DailyMed. Current Medication Information for BUTISOL SODIUM TABLETS (butabarbital sodium tablets) (July 2009). Available from, as of March 7, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10579
Allergic reactions that occur especially in persons who tend to have asthma, urticaria, angioedema, and similar conditions. /Barbiturates/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 362
For more Drug Warnings (Complete) data for Butabarbital (29 total), please visit the HSDB record page.
The toxic dose of barbiturates varies considerably. In general, an oral dose of 1 gram of most barbiturates produces serious poisoning in an adult. Death commonly occurs after 2 to 10 grams of ingested barbiturates.
US Natl Inst Health; DailyMed. Current Medication Information for BUTISOL SODIUM (butabarbital sodium tablets) (July 2009). Available from, as of March 7, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10579
Potentially lethal blood concentrations are those in excess of 80 ug/mL for phenobarbital, 50 ug/mL for amobarbital or butabarbital, and approximately 30 ug/mL for secobarbital or pentobarbital; however, some patients have survived much higher blood concentrations. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2578
Butabarbital is indicated for use as a sedative or hypnotic. Butabarbital should not be used to treat insomnia for longer than 2 weeks.
Butabarbital potentiates GABAergic neurons while inhibiting neuronal acetylcholine and glutamate receptors to produce sedation. Butabarbital is an intermediate acting barbiturate with a duration of action of approximately 6-8 hours. The therapeutic index is quite wide as doses vary considerably from patient to patient. Patients should be counselled regarding the risk of worsening insomnia, drowsiness, falls, and complex behaviour while not fully awake.
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
Route of Elimination
Barbiturates such as butabarbital are predominantly eliminated in the urine. In dogs, 3-5% of the dose is eliminated in the urine as the unchanged parent compound.
Butabarbital sodium tablets and butabarbital sodium oral solution, is the sodium salt of a weak acid. Barbiturates are weak acids that are absorbed and rapidly distributed to all tissues and fluids with high concentrations in the brain, liver, and kidneys. Barbiturates are bound to plasma and tissue proteins. The rate of absorption is increased if it is ingested as a dilute solution or taken on an empty stomach.
US Natl Inst Health; DailyMed. Current Medication Information for BUTISOL SODIUM TABLETS (butabarbital sodium tablets) (July 2009). Available from, as of March 7, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10579
Butabarbital sodium is absorbed from the GI tract. Peak plasma concentrations are achieved within 3-4 hours following oral administration of the drug. Plasma butabarbital concentrations of 2-3 ug/mL produce sedation, and plasma concentrations of 25 ug/mL produce sleep in most patients. Plasma butabarbital concentrations of greater than 30 ug/mL may produce coma, and those in excess of 50 ug/mL are potentially lethal.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2581
Approximately 1-2% of an oral dose /of butabarbital/ is excreted unchanged in the urine.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2581
Fluid & tissue specimens collected from 30 subjects at autopsy were assayed for amylobarbitone (amobarbital), butobarbitone (butethal), pentobarbitone (pentobarbital), quinalbarbitone (secobarbital) and the corresponding hydroxylated metabolites by gas liquid chromatography. Where one barbiturate was ingested, an inverse relationship between lipid solubility of the drug and the distribution in fluids and tissues was observed. In most cases the liver, and in the remainder the spleen, contained the highest concn of barbiturate. Bile concn were often in excess of those in the corresponding liver. The metabolites of the 4 sedative barbiturates were usually present in lower amounts than the parent drugs in the fluids and tissues of most subjects but urine often contained much higher concn of metabolites, sometimes exceeding that of the parent drug in the liver. Admin of 2 or more barbiturates together did not appear to affect the distribution and metabolism of the individual drugs.
Robinson AE; McDowall RD; J Pharm Pharmacol 31: 357-65 (1979)
For more Absorption, Distribution and Excretion (Complete) data for Butabarbital (13 total), please visit the HSDB record page.
Data regarding the metabolism of butabarbital in humans are not readily available. In dogs, butabarbital undergoes metabolism to a final glucuronide metabolite.
Butabarbital is metabolized in the liver by oxidation of the sec-butyl substituent at C5 to form 5-ethyl-5-(1-methyl-2-carboxyethyl) barbituric acid, an inactive metabolite, which is then excreted in the urine.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2581
Most barbiturates are transformed in body to inactive metabolites. Principal site of biotransformation is liver. /Barbiturates/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 115
Barbiturates are transformed by 4 routes: 1. Oxidation of radical at C5. This is by far most important pathway ... Products are ... polar alcohols, ketones, phenols, or carboxylic acids ... /Barbiturates/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 116
With the exception of the less lipid-soluble aprobarbital and phenobarbital, nearly complete metabolism and/or conjugation of barbiturates in the liver precedes their renal excretion. The oxidation of radicals at C5 is the most important biotransformation responsible for termination of biological activity. Oxidation results in the formation of alcohols, kentones, phenols, or carboxylic acids, which may appear in the urine as such or as glucuronic acid conjugates. In some instances (eg, phenobarbital), N-glucosylation is an important metabolic pathway. Other biotransformations include N-hydroxylation, desulfuration of thiobarbiturates to oxybarbiturates, opening of the barbituric acid ring, and N-dealkylation of N-alkylbarbiturates to active metabolites (eg, mephobarbital to phenobarbital).
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 361
Butabarbital has a half life of 100 hours but its duration of action is only 6-8 hours.
The average plasma half-life for butabarbital is 100 hours in the adult.
US Natl Inst Health; DailyMed. Current Medication Information for BUTISOL SODIUM TABLETS (butabarbital sodium tablets) (July 2009). Available from, as of March 7, 2010: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=10579
The elimination half-life of butabarbital was reportedly 34-42 hours in one study; one manufacturer states that the plasma half-life averages 100 hours in adults.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2581
Barbiturates like butabarbital potentiate GABA-A receptors and inhibit receptors for neuronal acetylcholine, and kainate. GABA-A receptors are predominantly on the post-synaptic membrane, and upon activation, open chloride channels to hyperpolarize the neuron and decreased firing rate. Potentiation of GABAergic neurons produces sedation. Inhibition of neuronal acetylcholine receptors and glutamate receptors of the kainate subtype desensitize their respective neurons, producing sedation.
The exact mechanism(s) by which barbiturates exert their effect on the CNS, has not been fully elucidated. However, it is believed that such effects are related, at least partially, to the drugs' ability to enhance the activity of gamma-aminobutyric acid (GABA), the principal inhibitory neurotransmitter in the CNS, by altering inhibitory synaptic transmissions that are mediated by GABAA receptors. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Although the drugs act throughout the CNS, a site of particular sensitivity is the polysynaptic midbrain reticular formation which is concerned with the arousal mechanism. Barbiturates induce an imbalance in central inhibitory and facilitatory mechanisms influencing the cerebral cortex and the reticular formation. The significance of the effect of barbiturates on neurotransmitters is unclear. It appears that the drugs decrease the excitability of both presynaptic and postsynaptic membranes. It has not been determined which of the various actions of barbiturates at cellular and synaptic levels are responsible for their sedative and hypnotic effects. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Relatively low doses of the barbiturates depress the sensory cortex, decrease motor activity, and produce sedation and drowsiness. In some patients, however, drowsiness may be preceded by a period of transient elation, confusion, euphoria, or excitement, especially after subhypnotic doses of aprobarbital, pentobarbital, or secobarbital. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Larger doses distort judgment, cloud perception, suppress motor activity, and produce drowsiness and sleep. Still larger doses induce anesthesia. Barbiturate-induced sleep differs from physiologic sleep. Barbiturates reduce the rapid eye movement (REM) or dreaming stage of sleep. Stages III and IV sleep are also decreased. Although tolerance develops to the REM-suppressant effects during chronic administration, REM rebound occurs when the drugs are withdrawn, and the patient may experience markedly increased dreaming, nightmares, and/or insomnia. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
For more Mechanism of Action (Complete) data for Butabarbital (13 total), please visit the HSDB record page.