
Synopsis
Synopsis
0
CEP/COS
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
FDA Orange Book

0
Europe

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
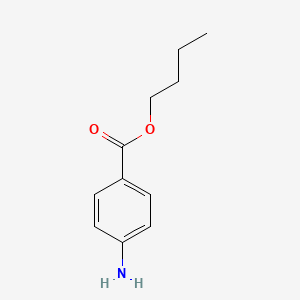

1. Butamben
2. Butamben Picrate
3. Butanylcaine
4. Butesin
5. Butyl Aminobenzoate
6. Butylcaine
7. N-butyl-p-aminobenzoate
8. Zyljectin
1. Butamben
2. 94-25-7
3. Butesin
4. Butyl Aminobenzoate
5. Butoform
6. Butylcaine
7. Butesine
8. Planoform
9. Scuroform
10. Scuroforme
11. Butyl P-aminobenzoate
12. 4-(butoxycarbonyl)aniline
13. Butyl Keloform
14. Benzoic Acid, 4-amino-, Butyl Ester
15. N-butyl P-aminobenzoate
16. P-aminobenzoic Acid Butyl Ester
17. 4-aminobenzoic Acid Butyl Ester
18. 4-aminobenzoic Acid N-butyl Ester
19. N-butyl-p-aminobenzoate
20. Butyl Paba
21. Benzoic Acid, P-amino-, Butyl Ester
22. Mfcd00017112
23. Nsc 128464
24. 4-amino Butylbenzoate
25. P-aminobenzoic Acid, Butyl Ester
26. Butylester Kyseliny P-aminobenzoove
27. 4-aminobenzoic Acid Butyl
28. Nsc-128464
29. Mls000028721
30. Chebi:3231
31. Efw857872q
32. Nsc128464
33. Cas-94-25-7
34. Ncgc00016353-03
35. Smr000059139
36. Dsstox_cid_2417
37. Dsstox_rid_76583
38. Dsstox_gsid_22417
39. Butamben [usan]
40. Butsein
41. Ccris 5891
42. Hsdb 4245
43. Butamben [usan:usp]
44. Sr-01000721933
45. Einecs 202-317-1
46. Brn 1211465
47. Butoforme
48. Unii-efw857872q
49. Ai3-02284
50. Butylester Kyseliny P-aminobenzoove [czech]
51. Butamben (usp)
52. Prestwick_994
53. Butesin (tn)
54. Butyl -4-aminobenzoate
55. Spectrum_000025
56. Butamben [hsdb]
57. N-butyl 4-aminobenzoate
58. Opera_id_633
59. Specplus_000829
60. Butamben [mi]
61. Butamben [vandf]
62. Prestwick0_000761
63. Prestwick1_000761
64. Prestwick2_000761
65. Prestwick3_000761
66. Spectrum2_000850
67. Spectrum3_001848
68. Spectrum4_000832
69. Spectrum5_001496
70. Butamben [usp-rs]
71. Butyl Paba [inci]
72. Wln: Zr Dvo4
73. Schembl81735
74. Bspbio_000802
75. Bspbio_003236
76. Kbiogr_001403
77. Kbioss_000385
78. Mls002303044
79. Bidd:er0674
80. Divk1c_000838
81. Divk1c_006925
82. Spectrum1500767
83. Spbio_000839
84. Spbio_002741
85. Bpbio1_000884
86. Chembl127516
87. Butamben [usp Monograph]
88. Dtxsid7022417
89. Hms502j20
90. Kbio1_000838
91. Kbio1_001869
92. Kbio2_000385
93. Kbio2_002953
94. Kbio2_005521
95. Kbio3_002736
96. Ninds_000838
97. P-aminobenzoic Acid N-butyl Ester
98. Abbott-34842 Free Base
99. Hms1570i04
100. Hms1921g20
101. Hms2092k06
102. Hms2097i04
103. Hms2234m07
104. Hms3372c17
105. Hms3714i04
106. Hms3885n08
107. Pharmakon1600-01500767
108. Butyl Aminobenzoate [mart.]
109. Hy-b1430
110. Zinc1530937
111. Tox21_110392
112. Tox21_200378
113. Butyl Aminobenzoate [who-dd]
114. Ccg-40317
115. Nsc757433
116. S4583
117. Stl169355
118. Akos000119787
119. Tox21_110392_1
120. Cs-4822
121. Db11148
122. Nsc-757433
123. Idi1_000838
124. Ncgc00016353-01
125. Ncgc00016353-02
126. Ncgc00016353-04
127. Ncgc00016353-05
128. Ncgc00016353-06
129. Ncgc00016353-08
130. Ncgc00091154-01
131. Ncgc00091154-02
132. Ncgc00091154-03
133. Ncgc00091154-04
134. Ncgc00257932-01
135. Ac-13349
136. Ds-13802
137. Para-aminobenzoic Acid Butyl Ester
138. Sy051932
139. Sbi-0051887.p002
140. Db-029553
141. A0270
142. Ab00052407
143. Ft-0617559
144. A16090
145. C07875
146. D00730
147. W16695
148. Ab00052407_12
149. Ae-641/05573061
150. Q-200433
151. Q5002348
152. Sr-01000721933-2
153. Sr-01000721933-3
154. Brd-k27217864-001-04-1
155. Brd-k27217864-001-05-8
156. Brd-k27217864-001-15-7
157. Z57126994
158. F2190-0449
159. Butamben, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 193.24 g/mol |
|---|---|
| Molecular Formula | C11H15NO2 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 5 |
| Exact Mass | 193.110278721 g/mol |
| Monoisotopic Mass | 193.110278721 g/mol |
| Topological Polar Surface Area | 52.3 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 174 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anesthetics, Local
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
... POORLY SOL IN WATER &, CONSEQUENTLY, TOO SLOWLY ABSORBED TO BE TOXIC. THEY CAN BE APPLIED DIRECTLY TO WOUNDS & ULCERATED SURFACES WHERE THEY REMAIN LOCALIZED FOR LONG PERIODS OF TIME ... ACCOUNTS FOR SUSTAINED ANESTHETIC ACTION. ... MOST IMPORTANT MEMBERS OF SERIES ARE ... BUTAMBEN, USP (BUTYL AMINOBENZOATE, BUTESIN).
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 310
MEDICATION (VET): TOPICALLY, AS SPRAY OR IN OINTMENTS (1-2%). ...PARENTERAL USE IN OIL HAS PROVIDED ANESTHESIA FOR UP TO 1 OR 2 DAYS & IS OCCASIONALLY USED IN DEEP PERIANAL INJECTIONS (OR WITH PROCAINE BASE & BENZYL ALC) WHERE PROLONGED PROTECTION AGAINST STRAINING IS DESIRED.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 55
Topical anesthetics are indicated to relieve pain, pruritus, and inflammation associated with minor skin disorders, including: burns, minor, including sunburn; bites (or stings), insect; dermatitis, contact, including poison ivy, poison oak, or poison sumac; wounds, minor such as cuts and scratches. /Included in US product labeling; Topical anesthetics/
USP. Convention. USPDI - Drug Information for the Health Care Professional. 19th ed. Volume I.Micromedex, Inc. Englewood, CO., 1999. Content Prepared by the U.S. Pharmacopieal Convention, Inc., p. 155
Butamben was indicated for the treatment of chronic pain due to its long-duration effect. It is also indicated as a surface anesthetic for skin a mucous membrane and for the relief of pain and pruritus associated with anorectal disorders.
Butamben has been shown to selectively inhibit dorsal root pain signal transmission for periods of months when administered as epidural suspensions. The effect of butamben is not related to any significant loss of motor function which indicates that it targets specifically the pain-sensing C fibers of the dorsal root. When administered topically, butamben produced anesthesia by accumulating in the nerve cell membrane causing it to expand and lose its ability to depolarize and blocking the impulse transmission.
Anesthetics, Local
Drugs that block nerve conduction when applied locally to nerve tissue in appropriate concentrations. They act on any part of the nervous system and on every type of nerve fiber. In contact with a nerve trunk, these anesthetics can cause both sensory and motor paralysis in the innervated area. Their action is completely reversible. (From Gilman AG, et. al., Goodman and Gilman's The Pharmacological Basis of Therapeutics, 8th ed) Nearly all local anesthetics act by reducing the tendency of voltage-dependent sodium channels to activate. (See all compounds classified as Anesthetics, Local.)
Absorption
When butamben is administered epidurally in a suspension form, the physical characteristics of butamben allow a very slow release. When administered topically, butamben is also reported to have a very low systemic absorption which allows for a longer duration of action.
Route of Elimination
The metabolites found in plasma after cholinesterase processing are disposed of in the urine.
Volume of Distribution
This pharmacokinetic property has not been determined.
Clearance
Clearance is flow-limited and it highly depends on the state of protein-bound form.
The metabolic pathway of butamben follows the same pattern of other local anesthetics and it is driven mainly by the hydrolysis via cholinesterase for the formation of inert metabolites.
The effective half-life of unencapsulated butamben is registered to be of 90 minutes. Some efforts were made to prepare D, L-lactic acid capsules which increased the half-life of butamben to even 400 hours.
Butamben acts by inhibiting the voltage-gated calcium channels in dorsal root ganglion neurons. The modification in this channels is thought to cause a disturbance of the channel kinetics acceleration. It is reported as well that butamben is an inhibitor of the sodium channels and a delayed rectifier of potassium currents. All the effects of butamben are performed in the root ganglion neurons which suggests that the related anesthetic effect may be caused by the reduced electrical excitability.
...ACTION IS TO INTERFERE WITH INITIATION & TRANSMISSION OF NERVE IMPULSE. PRESENT THEORY HOLDS THAT LOCAL ANESTHETICS PREVENT DEPOLARIZATION OF NERVE MEMBRANE &, HENCE, PROPAGATION OF IMPULSE. ...THOUGHT TO BE DUE TO INTERFERENCE WITH MUTUAL EXCHANGE OF SODIUM & POTASSIUM IONS ACROSS MEMBRANE. /LOCAL ANESTHETICS/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 987
Related Excipient Companies

Excipients by Applications
Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
99
PharmaCompass offers a list of Butamben API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Butamben manufacturer or Butamben supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Butamben manufacturer or Butamben supplier.
PharmaCompass also assists you with knowing the Butamben API Price utilized in the formulation of products. Butamben API Price is not always fixed or binding as the Butamben Price is obtained through a variety of data sources. The Butamben Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Butamben manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Butamben, including repackagers and relabelers. The FDA regulates Butamben manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Butamben API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Butamben manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Butamben supplier is an individual or a company that provides Butamben active pharmaceutical ingredient (API) or Butamben finished formulations upon request. The Butamben suppliers may include Butamben API manufacturers, exporters, distributors and traders.
click here to find a list of Butamben suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Butamben DMF (Drug Master File) is a document detailing the whole manufacturing process of Butamben active pharmaceutical ingredient (API) in detail. Different forms of Butamben DMFs exist exist since differing nations have different regulations, such as Butamben USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Butamben DMF submitted to regulatory agencies in the US is known as a USDMF. Butamben USDMF includes data on Butamben's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Butamben USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Butamben suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Butamben Drug Master File in Japan (Butamben JDMF) empowers Butamben API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Butamben JDMF during the approval evaluation for pharmaceutical products. At the time of Butamben JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Butamben suppliers with JDMF on PharmaCompass.
Butamben Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Butamben GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Butamben GMP manufacturer or Butamben GMP API supplier for your needs.
A Butamben CoA (Certificate of Analysis) is a formal document that attests to Butamben's compliance with Butamben specifications and serves as a tool for batch-level quality control.
Butamben CoA mostly includes findings from lab analyses of a specific batch. For each Butamben CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Butamben may be tested according to a variety of international standards, such as European Pharmacopoeia (Butamben EP), Butamben JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Butamben USP).
